
Exhibit 99.1

180 Life Sciences Corp. www.180lifesciences.com Corporate Presentation February 2023 Leading Research into Solving One of the World’s Largest Drivers of Disease: INFLAMMATION NASDAQ: ATNF

180 Life Sciences Corp. www.180lifesciences.com This Presentation is for informational purposes only and does not constitute an offer to sell, a solicitation of an offer to buy, or a recommendation to purchase any equity, debt or other financial instruments of 180 LIFE SCIENCES Corp . (“ 180 Life Sciences” or the “Company”) or any of its affiliates . This Presentation has been prepared to assist interested parties in making their own evaluation with respect to the business of 180 LIFE SCIENCES and for no other purpose . The information contained herein does not purport to be all - inclusive . The data contained herein is derived from various internal and external sources . No representation is made as to the reasonableness of the assumptions made within or the accuracy or completeness of any projections or any other information contained herein . Any data on past performance or projections contained herein is no indication as to future performance . 180 LIFE SCIENCES assumes no obligation to update the information in this Presentation . Forward - Looking Statements This Presentation includes “forward - looking statements” within the meaning of U . S . securities laws . Words such as “expect,” “estimate,” “project,” “budget,” “forecast,” “anticipate,” “intend,” “plan,” “may,” “will,” “could,” “should,” “believes,” “predicts,” “potential,” “continue” and similar expressions are intended to identify such forward - looking statements . These forward - looking statements involve significant risks and uncertainties that could cause the actual results to differ materially from the expected results and, consequently, you should not rely on these forward - looking statements as predictions of future events . These forward - looking statements and factors that may cause such differences include, without limitation, statements relating to the company’s continued listing on the Nasdaq Stock Market ; expectations regarding the future capitalization, resources and ownership structure of the company ; the inability to recognize the anticipated benefits of the business, which may be affected by, among other things, the ability of the company to execute its plans to develop and market new drug products and the timing, costs and results of these development programs ; estimates of the size of the markets for the company’s potential drug products ; potential litigation involving the company ; the validity or enforceability of the company’s intellectual property, including any challenges thereto ; global economic conditions ; geopolitical events and regulatory changes ; access to additional financing ; the duration and ongoing impact of the COVID - 19 pandemic ; and other risks and uncertainties indicated from time to time in the company’s filings with the Securities and Exchange Commission (the “SEC”) . The foregoing list of factors is not exclusive . Additional information concerning these and other risk factors is contained in the company’s most recent filings with the SEC . All subsequent written and oral forward - looking statements concerning the company and attributable to the company or any person acting on its behalf are expressly qualified in their entirety by the cautionary statements above . Readers are cautioned not to place undue reliance upon any forward - looking statements, which speak only as of the date made . The company does not undertake any obligation or undertaking to release publicly any updates or revisions to any forward - looking statement to reflect any change in its expectations or any change in events, conditions or circumstances on which any such statement is based . Disclaimer 2
180 Life Sciences Corp. www.180lifesciences.com 180 Life Sciences Overview Leading Research into Solving One of the World’s Largest Drivers of Disease: INFLAMMATION 3 Robust IP - Protected Product Pipeline with Large Market Potential • Three families of novel drugs address significant market opportunities in inflammation, fibrosis and pain with multiple programs in synchronized stages of development ⎼ Fibrosis & Anti - TNF ⎼ Synthetic CBD Analogs (SCAs) ⎼ a7nAChR • Strong IP Portfolio: 24 granted patents, 33 filed patent applications Scientific Pioneers Backed by Experienced Operators and Board • Founders: pioneers with 100+ cumulative years of discovery and clinical experience; successes include Remicade and Tysabri • Board: seasoned and diverse executives with broad skillsets that complement the Company’s needs • Senior Management: operators with decades of experience at large & small life sciences companies Numerous Near - Term Inflection Points • Anti - TNF programs: expecting to initiate a Phase 2 trial in Q2/Q3 2023 in POCD with initial data expected in Q2 2025 • SCA programs: validation ongoing, with a planned toxicity study
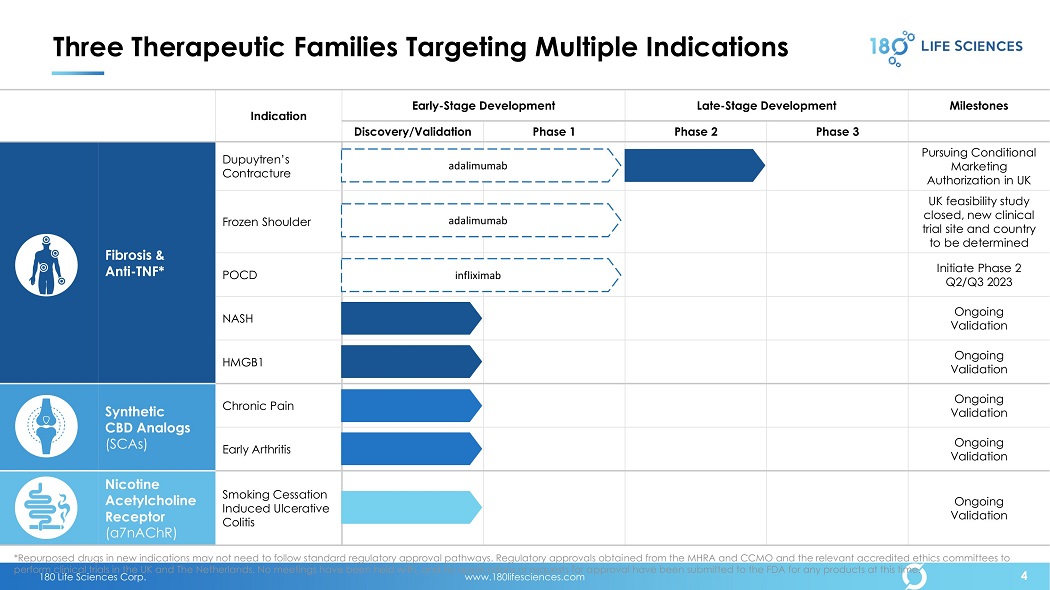
180 Life Sciences Corp. www.180lifesciences.com 4 Three Therapeutic Families Targeting Multiple Indications Indication Early - Stage Development Late - Stage Development Milestones Discovery/Validation Phase 1 Phase 2 Phase 3 Fibrosis & Anti - TNF* Dupuytren’s Contracture Pursuing Conditional Marketing Authorization in UK Frozen Shoulder UK feasibility study closed, new clinical trial site and country to be determined POCD Initiate Phase 2 Q2/Q3 2023 NASH Ongoing Validation HMGB1 Ongoing Validation Synthetic CBD Analogs (SCAs) Chronic Pain Ongoing Validation Early Arthritis Ongoing Validation Nicotine Acetylcholine Receptor ( α7 nAChR) Smoking Cessation Induced Ulcerative Colitis Ongoing Validation *Repurposed drugs in new indications may not need to follow standard regulatory approval pathways. Regulatory approvals obtai ned from the MHRA and CCMO and the relevant accredited ethics committees to perform clinical trials in the UK and The Netherlands. No meetings have been held with, and no applications or requests for a ppr oval have been submitted to the FDA for any products at this time. 4 adalimumab adalimumab infliximab

180 Life Sciences Corp. www.180lifesciences.com 5 Frank Knuettel II, MBA Independent Director Pamela Marrone, PhD Independent Director Prof. Larry Gold, PhD Independent Director Donald McGovern, Jr. Independent Director Russell Ray, MBA Independent Director Teresa DeLuca, MD, MBA Independent Director James Woody, MD, PhD Director, CEO Prof. Sir Marc Feldmann Executive Co - Chairman Co - Founder University of Oxford Prof. Lawrence Steinman Executive Co - Chairman Co - Founder Stanford University Jonathan Rothbard, PhD Chief Scientific Officer Prof. Jagdeep Nanchahal Co - Founder; Chair, Clinical Advisory Board University of Oxford Prof . Raphael Mechoulam Co - Founder Hebrew University Ozan Pamir Chief Financial Officer Management Team Board of Directors James Woody, MD, PhD Chief Executive Officer Prof. Sir Marc Feldmann Co - Chairman Prof. Lawrence Steinman Co - Chairman Experienced Leadership Team Founders
180 Life Sciences Corp. www.180lifesciences.com Fibrosis & Anti - TNF Therapeutics: Lead Clinical Indications Novel Treatments for TNF - Driven Conditions Dupuytren’s Contracture Frozen Shoulder POCD 6 • All three conditions are primarily driven by a pro - inflammatory protein called tumor necrosis factor (TNF) • Proof - of - Concept in Dupuytren’s Contracture has broader applications in Frozen Shoulder and POCD ⎯ No treatment options currently available that target and prevent early - stage fibrosis of the hand ⎯ Treating early - stage fibrosis can halt disease progression ⎯ Clinically significant Phase 2b data in Dupuytren’s Contracture, published in The Lancet Rheumatology ⎯ Phase 2b clinical data in Dupuytren’s Contracture provides a strong rationale to investigate anti - TNF treatment in Frozen Shoulder and POCD • Shorter development timeline for repurposing drugs ⎯ Can leverage previous studies and clinical data of anti - TNF approved drugs ⎯ Studies typically commence at Phase 2 and are potentially pivotal
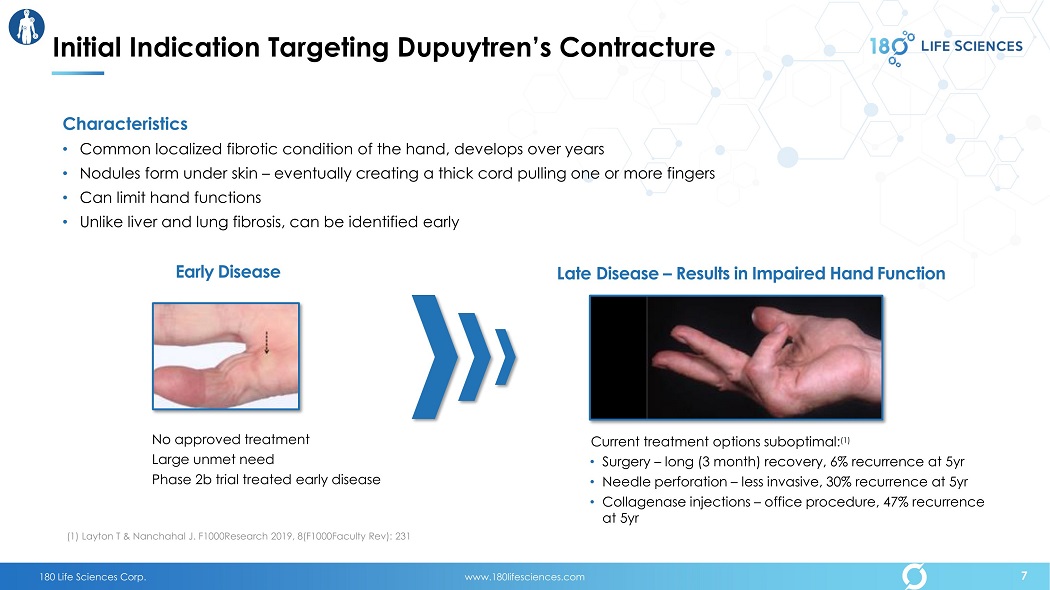
180 Life Sciences Corp. www.180lifesciences.com Characteristics • Common localized fibrotic condition of the hand , develops over years • Nodules form under skin – eventually creating a thick cord pulling one or more fingers • Can limit hand functions • Unlike liver and lung fibrosis, can be identified early Early D isease Late D isease – Results in Impaired H and Function No approved treatment Large unmet need Phase 2b trial treated early disease Current treatment options suboptimal: (1) • Sur gery – long (3 month) recovery, 6% recurrence at 5yr • N eedle perforation – less invasive, 30% recurrence at 5yr • C ollagenase injections – office procedure, 47% recurrence at 5 yr Initial Indication Targeting Dupuytren’s Contracture ( 1 ) Layton T & Nanchahal J. F1000Research 2019, 8(F1000Faculty Rev): 231 7
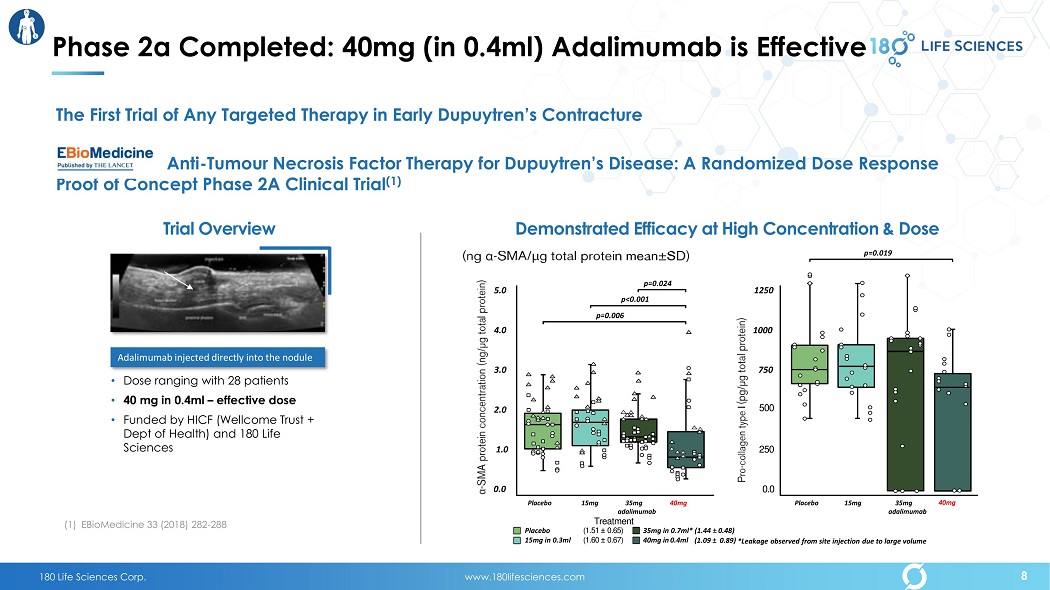
180 Life Sciences Corp. www.180lifesciences.com Phase 2a Completed: 40mg (in 0.4ml) Adalimumab is Effective • Dose ranging with 28 patients • 40 mg in 0.4ml – effective dose • Funded by HICF (Wellcome Trust + Dept of Health) and 180 Life Sciences Trial Overview Adalimumab injected directly into the nodule Demonstrated Efficacy at High C oncentration & D ose 15mg in 0.3ml (1.60 “ 0.67) 40mg in 0.4ml Placebo (1.51 “ 0.65) 35mg in 0.7ml* (1.44 “ 0.48) (1.09 “ 0.89) *Leakage observed from site injection due to large volume 5.0 4.0 3.0 2.0 1.0 0.0 Placebo 15mg 35mg 40mg a dalimumab Treatment p=0.024 p=0.006 p<0.001 1250 1000 750 500 250 0.0 Placebo 15mg 35mg 40mg a dalimumab p=0.019 The First Trial of Any Targeted Therapy in Early Dupuytren’s Contracture Anti - Tumour Necrosis Factor Therapy for Dupuytren’s Disease: A Randomized Dose Response Proof of Concept Phase 2A Clinical Trial (1) (1) EBioMedicine 33 (2018) 282 - 288 8
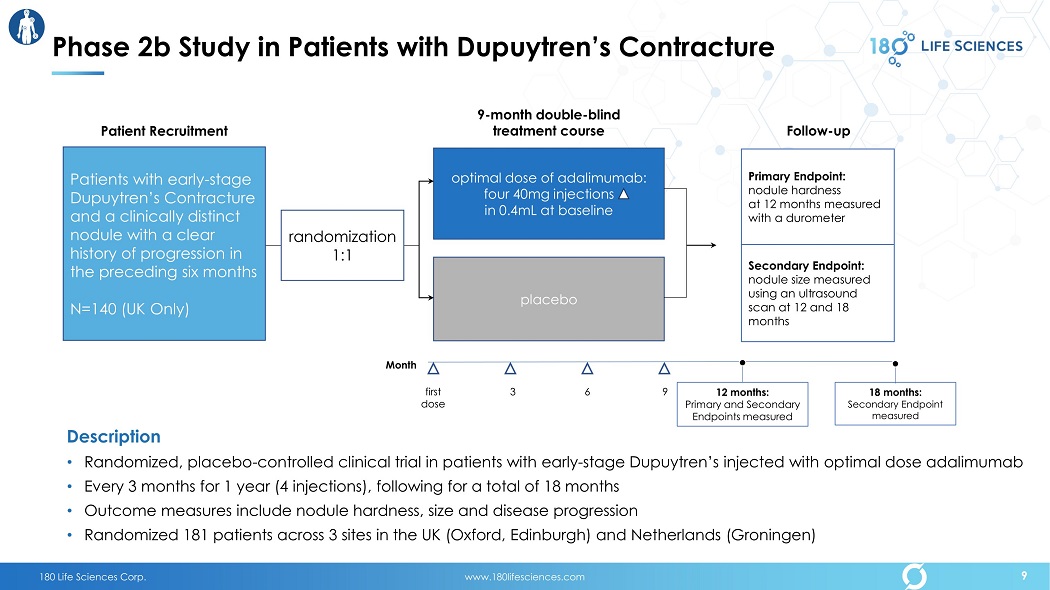
180 Life Sciences Corp. www.180lifesciences.com optimal dose of adalimumab: four 40mg injections in 0.4mL at baseline placebo Primary Endpoint: nodule hardness at 12 months measured with a durometer Month first dose 3 6 9 Secondary Endpoint: n odule size measured using an ultrasound scan at 12 and 18 months Follow - up 9 - month double - blind treatment course 12 months: Primary and Secondary Endpoints measured 18 months: Secondary Endpoint measured randomization 1:1 Patients with early - stage Dupuytren’s Contracture and a clinically distinct nodule with a clear history of progression in the preceding six months N=140 (UK Only) Patient Recruitment Description • Randomized, placebo - controlled clinical trial in patients with early - stage Dupuytren’s injected with optimal dose adalimumab • Every 3 months for 1 year (4 injections), following for a total of 18 months • Outcome measures include nodule hardness, size and disease progression • Randomized 181 patients across 3 sites in the UK (Oxford, Edinburgh) and Netherlands (Groningen) Phase 2b Study in Patients with Dupuytren’s Contracture 9
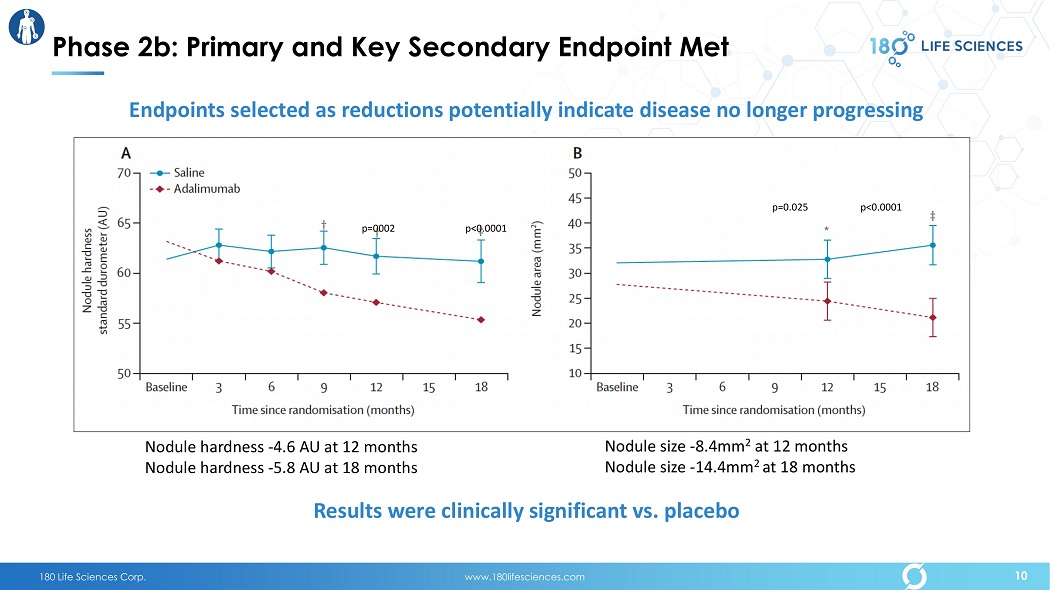
180 Life Sciences Corp. www.180lifesciences.com p=0002 p<0.0001 p<0.0001 p=0.025 Results were clinically significant vs. placebo Phase 2b: Primary and Key Secondary Endpoint Met Endpoints selected as reductions potentially indicate disease no longer progressing Nodule hardness - 4.6 AU at 12 months Nodule hardness - 5.8 AU at 18 months Nodule size - 8.4mm 2 at 12 months Nodule size - 14.4mm 2 at 18 months 10
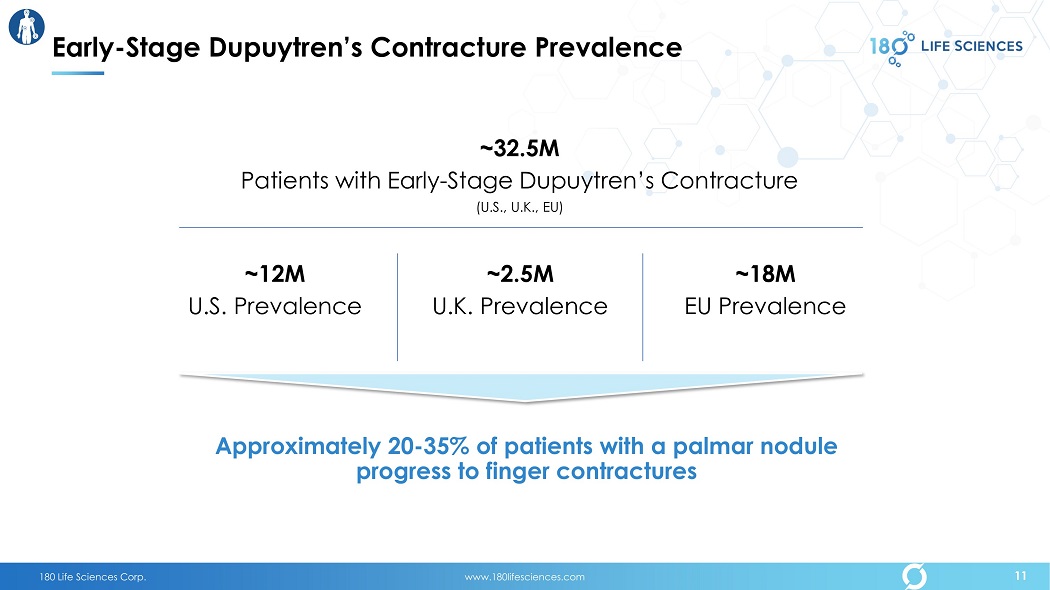
180 Life Sciences Corp. www.180lifesciences.com 11 Early - Stage Dupuytren’s Contracture Prevalence 11 ~32.5M Patients with Early - Stage Dupuytren’s Contracture (U.S., U.K., EU) ~12M U.S. Prevalence ~2.5M U.K. Prevalence ~18M EU Prevalence Approximately 20 - 35% of patients with a palmar nodule progress to finger contractures
180 Life Sciences Corp. www.180lifesciences.com • Over 300,000 hip fractures each year in the US alone (1) • Strong clinical evidence for anti - TNF as preventative therapy • Patent claims granted, patent is licensed from Kennedy Trust, UK • Phase 2 multi - center trial of pre - operative anti - TNF in hip fracture surgery planned to initiate by Q3 2023; single dose administered just prior to surgery; to be completed in 2 years Post Operative Delirium/ Cognitive Deficit (POCD) Frozen Shoulder • Affects 9% of the of the population aged 25 - 64yr, more common in diabetics (2) • Only treatment for early stage is local steroid injection for short term relief • Trial protocol completed and NIHR grant received for feasibility study. Feasibility study closed for enrollment • Phase 2 clinical trial site and country will be determined in 2023 (1) https://www.cdc.gov/homeandrecreationalsafety/falls/adulthipfx.html (2) Walker - Bone K et al (2004) Arthritis Rheum 51(4):642 - 651 12 Additional Near - Term Anti - TNF Indications
180 Life Sciences Corp. www.180lifesciences.com SCA Family: Synthetic CBD Analogs for Pain & Inflammation • Safe & non - psychoactive • Formulated to offer improved oral bioavailability (>3x) • Rigorously tested in clinical trials for inflammatory pain (efficacy and dosing) • Granted market approval by FDA, EMA and others • A real alternative to unregulated consumption of medical cannabis or OTC CBD (no clinical evidence, not FDA approved, unreliable composition, unpredictable dosing and safety) Challenges with Medical C annabis / OTC CBD 180 Life Sciences Solution Developing proprietary compounds which aim to be: 13 Variable composition, potency, and may contain undesirable contaminants Side effects can be triggered by THC (e.g., psychosis) Little clinical data from approved drugs exist (outside of epilepsy) to determine dosing Variable uptake and low absorption (~4 - 9%) due to lipophilic properties of CBD / CBD - like Use SYNTHETIC >99.5% pure SCAs Use synthetic CBD Analogs (SCAs) – no THC Planning blinded clinical trials initially in musculoskeletal pain and arthritis Developing novel, patented ProNanoLipospheres (PNL) which enhance bioavailability
180 Life Sciences Corp. www.180lifesciences.com a7nAChR Family: Novel Platform for Ulcerative Colitis a7nAChR is a nicotine acetylcholine receptor and a central factor in evolutionarily ancient neural circuit to control of inflammation (1,2) (1) Rothbard JB et al. Identification of a common immune regulatory pathway induced by small heat shock proteins, amyloid fibrils, and nicotine. Proc Natl Acad Sci U S A. 2018 115:7081 - 7086. (2) Tracey KJ. Reflex control of immunity. Nat Rev Immunol. (2009) 9:418 – 28 14 • Large pharma initially touted a7 as a pharmaceutical target for Alzheimer’s disease and schizophrenia ⎯ Multiple specific agonists developed ⎯ All shown to be safe, but did not meet milestones in human clinical trials • 180 Life Sciences aims to repurpose a7nAChR for inflammation ⎯ Nicotine binds a7 and is a known immune suppressive ⎯ A subgroup of patients who cease smoking subsequently acquire ulcerative colitis (a large, growing market: 2012 - $4.2B; 2022 - $6.6B) ⎯ Treatment has a high probability of therapeutic success (can be viewed as nicotine replacement therapy without issues of addi cti on) × Capability to induce remission is quite low × Known deleterious side effects of steroids Existing Therapies Sub - Optimal × Long - term administration of thiopurine may correlate with increased risk of lymphoma × Cyclosporine leads to kidney damage × Serious adverse events, such as opportunistic infections, including tuberculosis, as well as congestive heart failure in cardiopathic patients Anti - inflammatory drugs (5 - aminosalicylates, corticosteroids) Immunosuppressants Infliximab (anti - TNF) x Fewer opportunistic infections x Reduced risk of kidney damage x Higher anticipated success rate a7nAChR Competitive Advantages Better safety and efficacy Faster time to market Lower development costs Novel therapeutic target Targeted clinical trial x Repurposing drugs previously proven safe (targeted Alzheimer’s & Schizophrenia) x Drugs stimulate vagal nerve, leading to localized anti - inflammatory response, similar to nicotine’s MoA x First clinical trial targeting patients who ceased smoking and developed ulcerative colitis
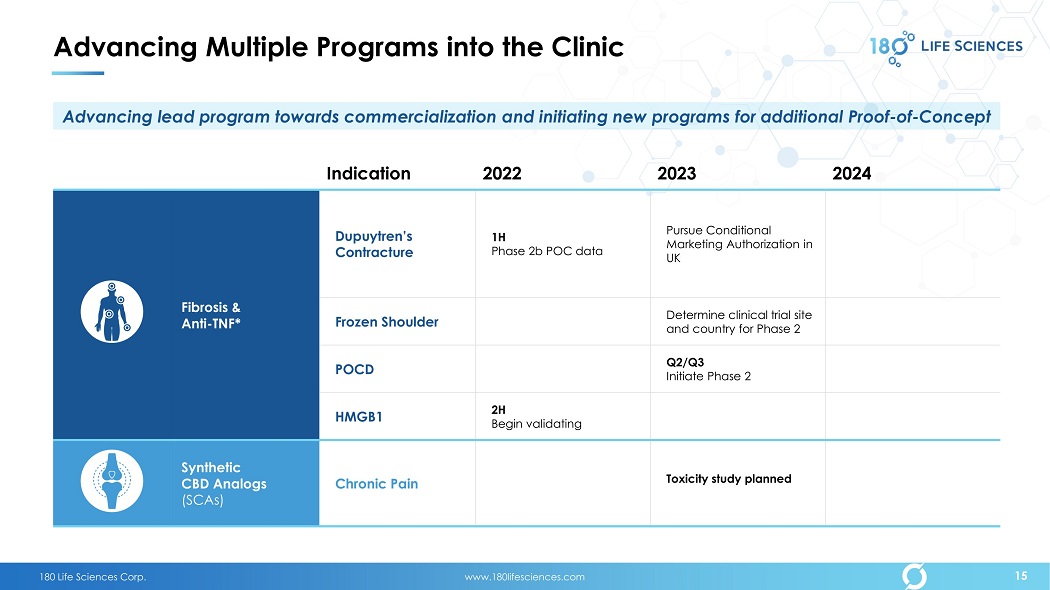
180 Life Sciences Corp. www.180lifesciences.com Advancing Multiple Programs into the Clinic Indication 2022 2023 2024 Fibrosis & Anti - TNF* Dupuytren’s Contracture 1H Phase 2b POC data Pursue Conditional Marketing Authorization in UK Frozen Shoulder Determine clinical trial site and country for Phase 2 POCD Q2/Q3 Initiate Phase 2 HMGB1 2H Begin validating Synthetic CBD Analogs (SCAs) Chronic Pain Toxicity study planned 15 Advancing lead program towards commercialization and initiating new programs for additional Proof - of - Concept
180 Life Sciences Corp. www.180lifesciences.com 16 180 Life Sciences Highlights Leading Research into Solving One of the World’s Largest Drivers of Disease: INFLAMMATION 16 Robust IP - Protected Product Pipeline with Large Market Potential Scientific Pioneers Backed by Experienced Operators and Board Numerous Near - Term Inflection Points

180 Life Sciences Corp. www.180lifesciences.com www.180lifesciences.com Thank you 17