
Exhibit 99.1

Corporate Presentation Q4 2020 Leading Research into Solving one of the World’s Largest Drivers of Disease: Inflammation
180 Life Sciences Corp. www.180lifesciences.com Q4 2020 2 This Presentation is for informational purposes only and does not constitute an offer to sell, a solicitation of an offer to buy , or a recommendation to purchase any equity, debt or other financial instruments of 180 LIFE SCIENCES Corp. (“180 Life Scie nce s”) or KBL Merger Corp. IV (“KBL”) or any of 180 Life Sciences’ or KBL’s affiliates’ securities. This Presentation has been prepared to assist interested parties in making their own evaluation with respect to the proposed business combination of 180 LIFE SCIENC ES and KBL and for no other purpose. The information contained herein does not purport to be all - inclusive. The data contained here in is derived from various internal and external sources. No representation is made as to the reasonableness of the assumptions made within or the accuracy or completeness of any projections or any other information contained herein. Any dat a o n past performance or projections contained herein is no indication as to future performance. 180 LIFE SCIENCES and KBL assume no obligation to update the information in this Presentation. Forward - Looking Statements This Presentation includes “forward - looking statements” within the meaning of U.S. federal securities laws. Words such as “expec t,” “estimate,” “project,” “budget,” “forecast,” “anticipate,” “intend,” “plan,” “may,” “will,” “could,” “should,” “believes, ” “predicts,” “potential,” “continue” and similar expressions are intended to identify such forward - looking statements. These forw ard - looking statements involve significant risks and uncertainties that could cause the actual results to differ materially from the expected results and, consequently, you should not rely on these forward - looking statements as predictions of future events. The se forward - looking statements and factors that may cause such differences include, without limitation, statements relating to the timing and completion of the proposed business combination; KBL’s continued listing on the Nasdaq Stock Market until clos ing of the proposed business combination; expectations regarding the capitalization, resources and ownership structure of the combined company; the inability to recognize the anticipated benefits of the proposed business combination, which may be affecte d by, among other things, the amount of cash available following redemptions by KBL stockholders; the ability to meet the Nasdaq Stock Market's listing standards following the consummation of the transactions contemplated by the proposed business com bination; costs related to the proposed business combination; expectations with respect to future performance, growth and anticipated acquisitions; ability to recognize the anticipated benefits of the proposed business combination; 180 Life Sc ien ces' ability to execute its plans to develop and market new drug products and the timing and costs of these development programs; 180 Life Sciences' estimates of the size of the markets for its potential drug products; potential litigation invol vin g KBL or 180 LIFE SCIENCES or the validity or enforceability of 180 Life Sciences’ intellectual property; global economic con dit ions; geopolitical events and regulatory changes; access to additional financing; and other risks and uncertainties indicated from tim e to time in filings with the Securities and Exchange Commission (the “SEC”). Other factors include the possibility that the proposed business combination does not close, including due to the failure to receive required security holder approvals, or the failure of other closing conditions. The foregoing list of factors is not exclusive. Additional information concerning these an d other risk factors is contained in KBL’s most recent filings with the SEC and will be contained in the proxy statement/prospe ctu s to be filed as result of the transactions described above. All subsequent written and oral forward - looking statements concerni ng KBL or 180 Life Sciences, the transactions described herein or other matters and attributable to KBL or 180 LIFE SCIENCES or any person acting on their behalf are expressly qualified in their entirety by the cautionary statements above. Readers are cauti on ed not to place undue reliance upon any forward - looking statements, which speak only as of the date made. None of KBL or 180 LIFE S CIENCES undertake or accept any obligation or undertaking to release publicly any updates or revisions to any forward - looking statement to reflect any change in their expectations or any change in events, conditions or circumstances on which a ny such statement is based. Additional Information and Where to Find It KBL has filed a registration statement on Form S - 4, which includes a preliminary proxy statement/prospectus for KBL’s stockholde rs, with the SEC. KBL’s definitive proxy statement/prospectus will be mailed to KBL’s stockholders that do not opt to receive th e document electronically. KBL and 180 LIFE SCIENCES urge investors, stockholders and other interested persons to read the prel imi nary proxy statement/prospectus, as well as other documents that will be filed with the SEC, because these documents will contain important information about the proposed business combination transaction. Such persons can also read KBL’s Annual Re por t on Form 10 - K for the fiscal year ended December 31, 2019, for a description of the security holdings of its officers and directors and their respective interests as security holders in the consummation of the proposed business combination transac tio n. KBL’s definitive proxy statement/prospectus, which is included in the registration statement, will be mailed to stockholde rs of KBL as of a record date to be established. KBL’s stockholders will also be able to obtain a copy of such documents, withou t c harge, by directing a request to: KBL Merger Corp. IV, 30 Park Place, Suite 45E, New York, NY 10007 ; e - mail: admin@kblvc.com . These documents can also be obtained, without charge, at the SEC’s website ( http://www.sec.gov ). Participants in the Solicitation KBL and its directors and executive officers, may be deemed to be participants in the solicitation of proxies for the special me eting of KBL’s stockholders to be held to approve the proposed transactions in connection with the business combination. Information regarding the persons who may, under the rules of the SEC, be deemed participants in the solicitation of KBL’s st ock holders in connection with the proposed transactions are set forth in the preliminary proxy statement/prospectus included in the registration statement that was filed with the SEC on November 12, 2019, as it has been amended from time to time . You can find information about KBL’s executive officers and directors in its Annual Report on Form 10 - K for the fiscal year en ded December 31, 2019, which was filed with the SEC on April 7, 2020. You can obtain free copies of these documents from KBL usin g t he contact information above. Disclaimer This Presentation is not a proxy statement or solicitation of a proxy, consent or authorization with respect to any securitie s o r in respect of the proposed transaction and shall not constitute an offer to sell or a solicitation of an offer to buy the s ecu rities of KBL and 180 Life Sciences, nor shall there be any sale of any such securities in any state or jurisdiction in which such offe r, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of such state or jur isd iction. No offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Ac t o f 1933, as amended. Disclaimer
3 • Scientific team and founders are pioneers with proven track record in drug discovery from the University of Oxford, Hebrew University and Stanford University • Developing three families of novel drugs addressing significant market opportunities in inflammation, fibrosis and pain: ─ Fibrosis & Anti - TNF ─ Synthetic CBD Analogs (SCAs) ─ a7nAChR • Multiple programs in synchronized stages of development • Numerous near - term inflection points for anti - TNF programs: one program late stage 2b/3 trial, two additional clinical programs projected to start Q3/4 2021 ─ Initial clinical anti - TNF clinical trials funded by investments and grants (UK). ─ Regulatory approvals obtained from the UK Medicines and Healthcare Products Regulatory Agency (MHRA) and the Dutch Centrale Commissie Mensgebonden Onderzoek (CCMO) and the relevant accredited ethics committees to perform clinical trials in the UK and The Netherlands for anti - TNF products(1) • Strong IP portfolio with a long lifespan, providing coverage up to 2039 (1) No meetings have been held with, and no applications or requests for approval have been submitted to the FDA for any prod uct s at this time. 180 Life Sciences Highlights 180 Life Sciences Corp. www.180lifesciences.com Q4 2020

4 Leadership Prof Sir Marc Feldmann Co - Chairman • Pioneer of anti - TNF therapy, world’s biggest drug class ($40B USD pa) • Anti - TNF discovery eventually led to Centocor’s acquisition by J&J for $4.9 B USD • 7 International awards for Biomedical Innovation, including Crafoord and Lasker Awards , fellow of the Royal Society Prof Lawrence Steinman Co - Chairman • Discovered role of integrins, led to Natalizumab, highly effective treatment for MS and IBD • Tysabri sold to Biogen for $3.25B • Member of National Academy of Sciences, 4 International awards for Biomedical Innovation including Charcot Prize; founder of Centocor Prof Raphael Mechoulam Founder, 180 LS • Godfather of cannabinoid chemistry; discovered the body’s endocannabinoid system • Recipient of Israel Exact Sciences Prize, member of Israel Academy of Science and Humanities Dr. James N. Woody CEO • Discovered Remicade at Centocor • Founded Avidia and Proteolix , which were sold to Amgen • GP of Latterell Venture Partners • 25+ years of pharmaceutical research and management experience • General Manager of Roche Biosciences (Former Syntex ) Dr Jonathan Rothbard CSO • Stanford University, broad experience in small molecule development • Founder of 5 biotech companies; Amylin sold to AstraZeneca and BMS for $5.3B USD Prof Jagdeep Nanchahal CMO • Surgeon - scientist, leading 2b/3 trial funded by Wellcome Trust and UK Dept. of Health • Fellow of the Royal College of Surgeons; discovered new treatments for fibrosis 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
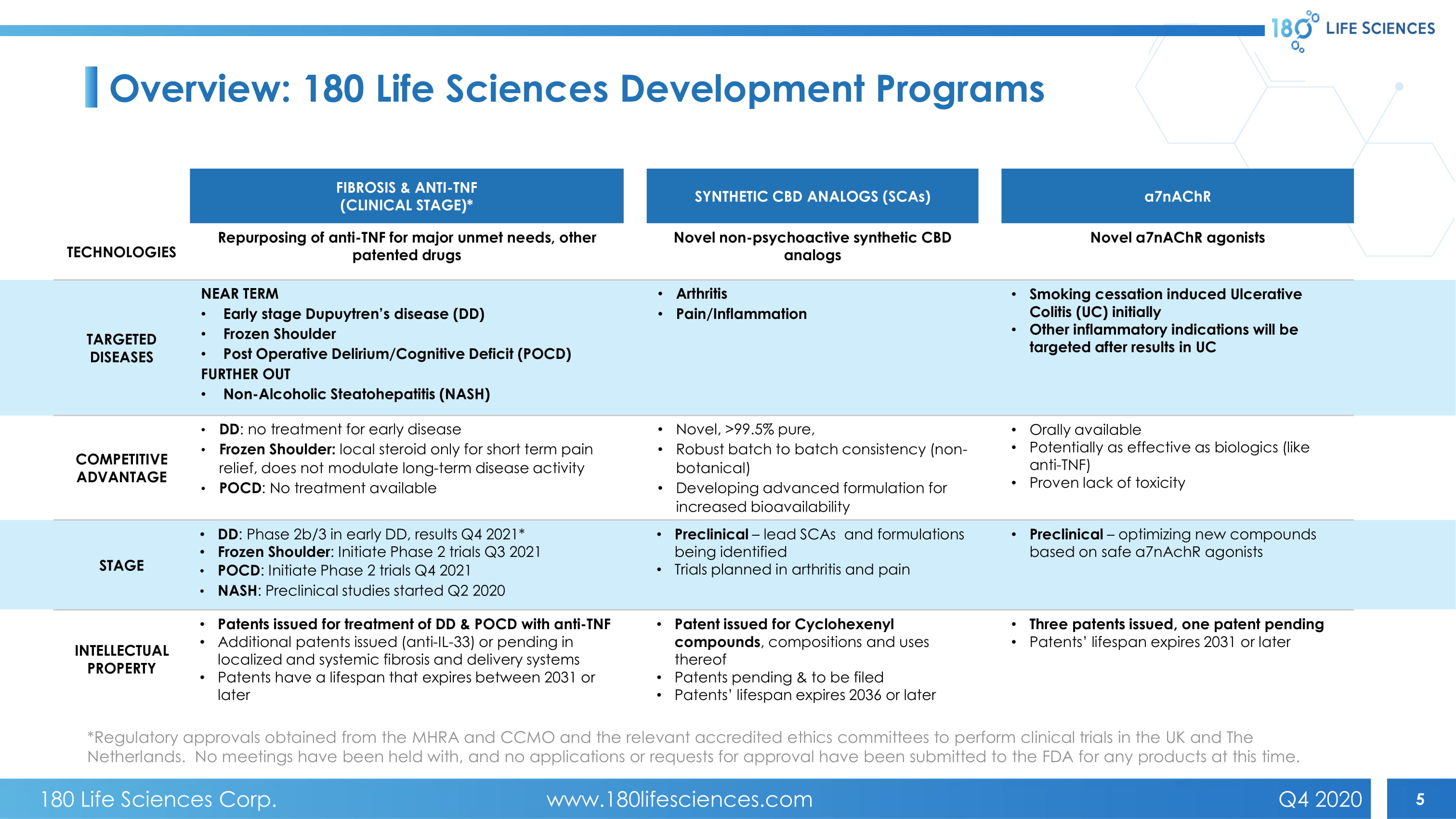
5 FIBROSIS & ANTI - TNF (CLINICAL STAGE)* SYNTHETIC CBD ANALOGS (SCAs) a7nAChR TECHNOLOGIES Repurposing of anti - TNF for major unmet needs, other patented drugs Novel non - psychoactive synthetic CBD analogs Novel α7 nAChR agonists TARGETED DISEASES NEAR TERM • Early stage Dupuytren’s disease (DD) • Frozen Shoulder • Post Operative Delirium/Cognitive Deficit (POCD) FURTHER OUT • Non - Alcoholic Steatohepatitis (NASH) • Arthritis • Pain/Inflammation • Smoking cessation induced Ulcerative Colitis (UC) initially • Other inflammatory indications will be targeted after results in UC COMPETITIVE ADVANTAGE • DD : no treatment for early disease • Frozen Shoulder: local steroid only for short term pain relief, does not modulate long - term disease activity • POCD : No treatment available • Novel, >99.5% pure, • Robust batch to batch consistency (non - botanical) • Developing advanced formulation for increased bioavailability • Orally available • Potentially as effective as biologics (like anti - TNF) • Proven lack of toxicity STAGE • DD : Phase 2b/3 in early DD, results Q4 2021* • Frozen Shoulder : Initiate Phase 2 trials Q3 2021 • POCD : Initiate Phase 2 trials Q4 2021 • NASH : Preclinical studies started Q2 2020 • Preclinical – lead SCAs and formulations being identified • Trials planned in arthritis and pain • Preclinical – optimizing new compounds based on safe a7nAchR agonists INTELLECTUAL PROPERTY • Patents issued for treatment of DD & POCD with anti - TNF • Additional patents issued (anti - IL - 33) or pending in localized and systemic fibrosis and delivery systems • Patents have a lifespan that expires between 2031 or later • Patent issued for Cyclohexenyl compounds , compositions and uses thereof • Patents pending & to be filed • Patents’ lifespan expires 2036 or later • Three patents issued, one patent pending • Patents’ lifespan expires 2031 or later *Regulatory approvals obtained from the MHRA and CCMO and the relevant accredited ethics committees to perform clinical trials in the UK and The Netherlands. No meetings have been held with, and no applications or requests for approval have been submitted to the FDA fo r a ny products at this time. Overview: 180 Life Sciences Development Programs 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
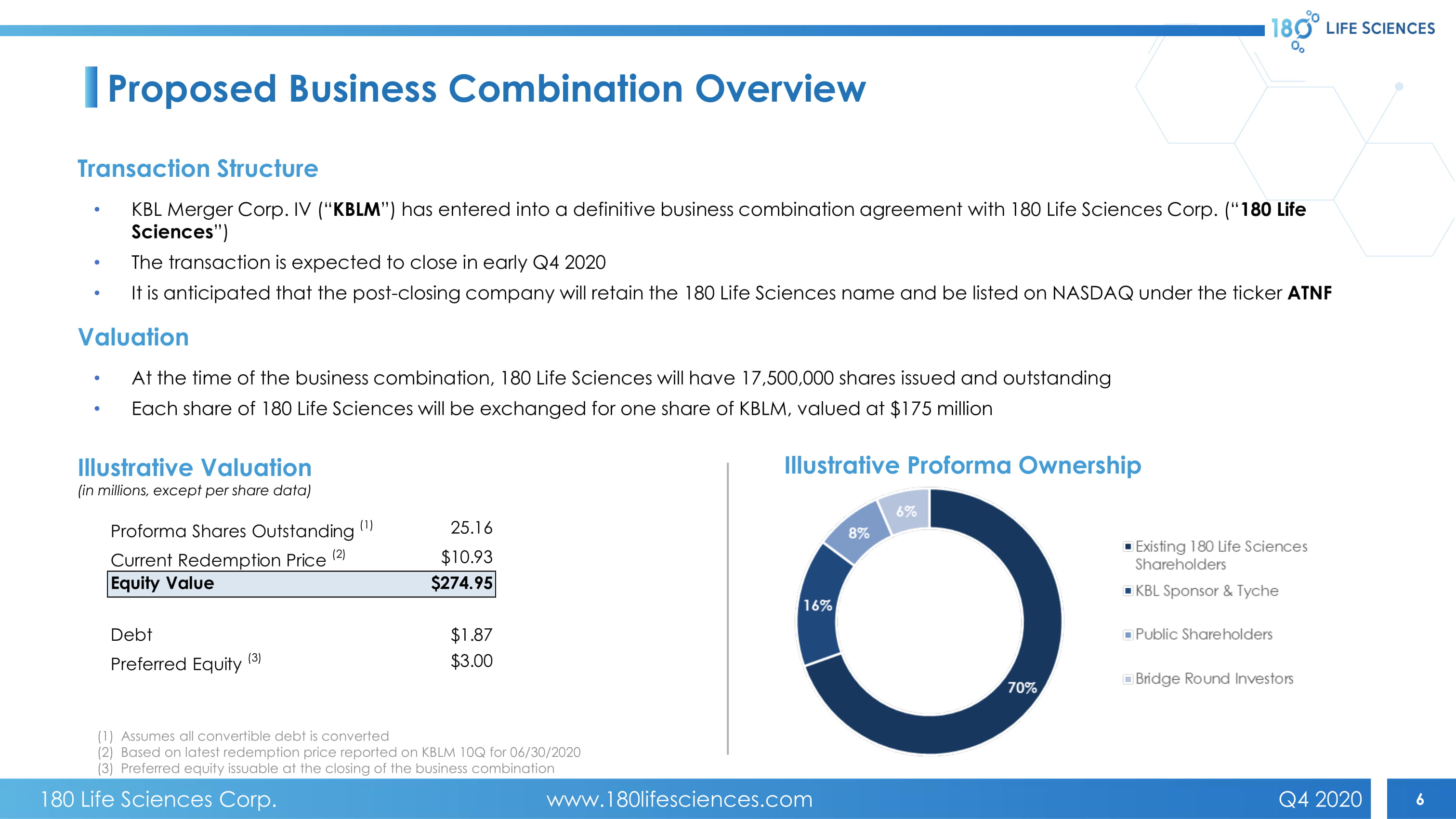
6 Proposed Business Combination Overview • KBL Merger Corp. IV (“ KBLM ”) has entered into a definitive business combination agreement with 180 Life Sciences Corp. (“ 180 Life Sciences ”) • The transaction is expected to close in early Q4 2020 • It is anticipated that the post - closing company will retain the 180 Life Sciences name and be listed on NASDAQ under the ticker ATNF Transaction Structure • At the time of the business combination, 180 Life Sciences will have 17,500,000 shares issued and outstanding • Each share of 180 Life Sciences will be exchanged for one share of KBLM, valued at $175 million Valuation Illustrative Valuation (in millions, except per share data) Illustrative Proforma Ownership (1) Assumes all convertible debt is converted (2) Based on latest redemption price reported on KBLM 10Q for 06/30/2020 (3) Preferred equity issuable at the closing of the business combination Proforma Shares Outstanding (1) 25.16 Current Redemption Price (2) $10.93 Equity Value $274.95 Debt $1.87 Preferred Equity (3) $3.00 180 Life Sciences Corp. www.180lifesciences.com Q4 2020

7 Management team has worked together on investments and transactions for 20 years Name Experience Current and Past Affiliations Marlene Krauss, M.D. CEO and Director 30+ years of experience in acquiring, growing and selling more than 20 companies in healthcare services, pharmaceuticals and medical devices 15+ years experience as an ophthalmic surgeon Founder and Managing Director of 3 KBL Healthcare venture capital funds Previously CEO and Chairman of three KBL SPACs - KBL Healthcare I, II, and III Board positions on over 10 healthcare companies including PneumRx , Lumenos , Summit Technology B.A. Cornell University, M.D. Harvard Medical School, M.B.A. Harvard Business School Joseph A. Williamson COO and Director 35+ years of experience as an operator and investor in the healthcare service business including senior living, home health and pharmacy distribution ( CCRx , National Homecare Holdings) Managing Partner at JAW Capital, an investment fund focusing on healthcare Co - founder and President of Concord Health Group acquired by KBL Acquisition I and subsequently sold to MultiCare B.S. Villanova University, J.D. Delaware Law School, M.B.A Temple University Experienced Leadership of KBL Merger Corp. IV 180 Life Sciences Corp. www.180lifesciences.com Q4 2020

8 Name Experience Current and Past Affiliations George Hornig Chairman 25+ years of senior operating, banking and investing experience CEO of RON Transatlantic Financial Holdings Former COO of Pine Bridge Investments, Credit Suisse Asset Management and Deutsche Bank (Americas) Co - founder and former COO of Wasserstein Perella & Co Former Director of KBL Acquisition Corp I A.B. , M.B.A., and J.D. Harvard University Sherrill Neff Director Founding partner of Quaker Partners, managing five life science venture funds with over $700 million in total assets Investor and/or Director of over 30 healthcare companies ( MedMark , Durata Therapeutics, Intact Vascular Inc.) Former President and COO of Neose Technologies Former Sr. Vice President of U.S. Healthcare B.A. Wesleyan University, J.D. University of Michigan Law School Andrew Sherman Director 20+ years in investment banking M&A and buyside roles Former Managing Director, Healthcare of Morgan Joseph Triartisan Previously worked on two SPACs: KBL Healthcare Acquisition Corp. III and Capitol Acquisition Corp. which completed a merger with Two Harbors Investment Corp. B.A. University of Pennsylvania, B.S. Wharton School of Business, M.B.A Harvard Business School EXPERIENCED MANAGEMENT TEAM AND BOARD CONT’D Experienced Leadership of KBL Merger Corp. IV (cont’d) 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
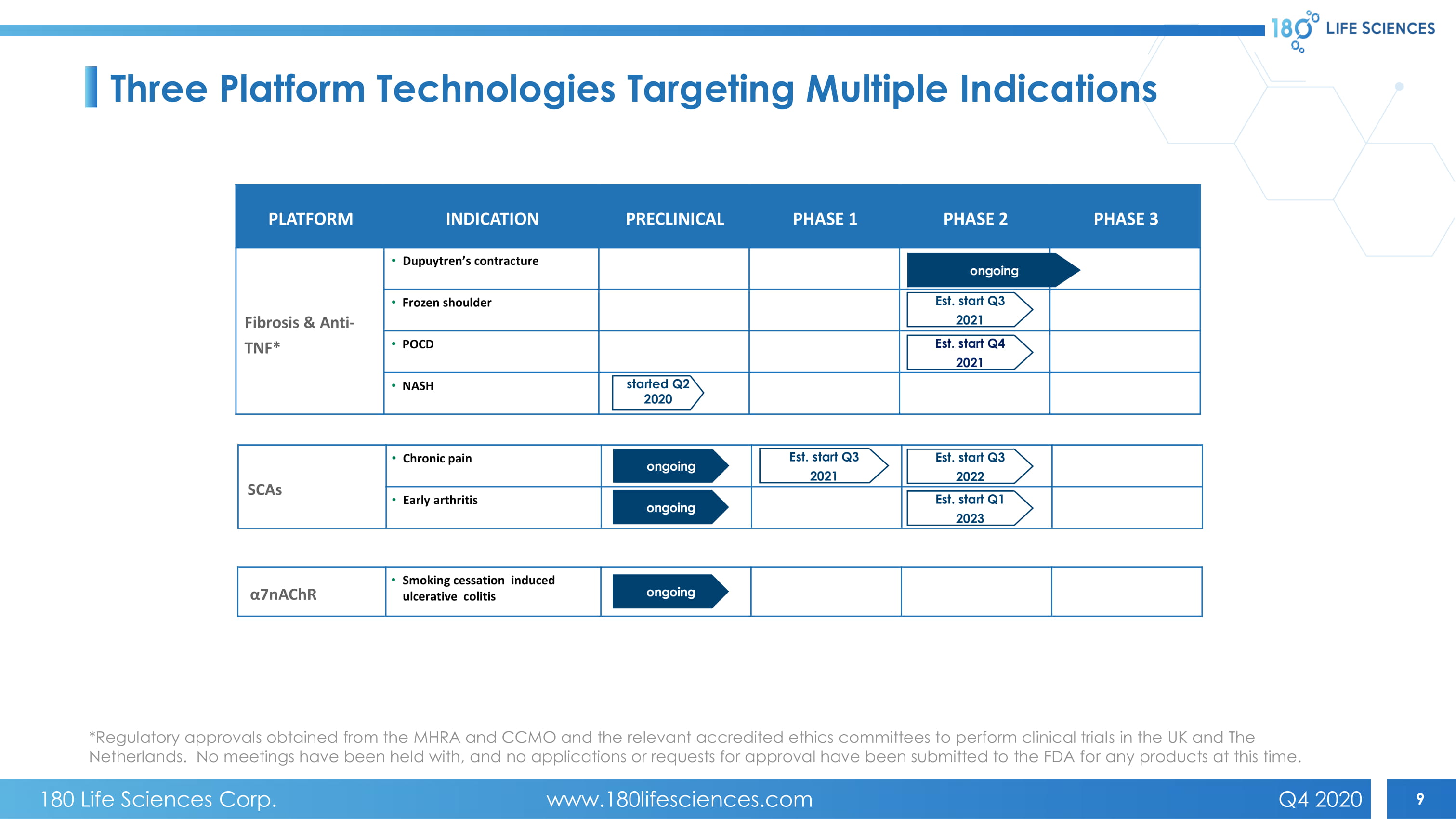
9 α7nAChR • Smoking cessation induced ulcerative colitis SCAs • Chronic pain • Early arthritis PLATFORM INDICATION PRECLINICAL PHASE 1 PHASE 2 PHASE 3 Fibrosis & Anti - TNF * • Dupuytren’s contracture • Frozen shoulder • PO C D • NASH Est. start Q1 2023 Est. start Q3 2022 started Q2 2020 Est. start Q3 2021 Est. start Q4 2021 ongoing ongoing ongoing ongoing Three Platform Technologies Targeting Multiple Indications Est. start Q3 2021 *Regulatory approvals obtained from the MHRA and CCMO and the relevant accredited ethics committees to perform clinical trial s i n the UK and The Netherlands. No meetings have been held with, and no applications or requests for approval have been submitted to the FDA fo r a ny products at this time. 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
10 Clinical Stage Lead Program: Fibrosis & Anti - TNF Targeting common diseases - facilitates trials and potential sales Clinical trials supported by Prof Sallie Lamb, UK Developing targeted therapies for: • Early Stage Dupuytren’s disease (DD) - patent issued; Phase 2b/3 results expected Q4 2021 (1) • Frozen shoulder - patent issued; clinical trials projected in Q3 2021 • Post operative cognitive decline (POCD) - patent issued, clinical trials projected in Q4 2021 • Liver fibrosis (NASH) - initial laboratory studies done with Celgene - BMS on human tissue; preclinical studies started Q2 2020 (1) Approval only from MHRA/CCMO and relevant accredited ethics committees. Dupuytren’s Disease Frozen Shoulder Nash POCD Program led by Profs Jagdeep Nanchahal & Sir Marc Feldmann, Oxford, and Dr Glenn Larsen, USA 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
11 Competitive Advantages • Expert Investigators ─ Established reputation in conducting clinical trials across academic and clinical networks (1) ─ Well practiced in publishing trials in peer reviewed clinical journals • Cost Effective ─ No payment for trial patients required in the UK/EU ─ Staff costs can be covered by academic grants ( Wellcome Trust, NIHR) • Shorter Timeline for Recruitment and Execution ─ Access to large registries of patients/diseases ─ Regulatory expertise in writing protocols, seeking approvals, conducting trials. Cost Effective, Time Efficient, Academic Led Clinical Trials Performed in UK (1) https://www.ndorms.ox.ac.uk/octru Developing the Only Treatment for Early Stage Fibrosis • Studies in DD lead the way for novel approach to develop clinical programs in other fibrotic diseases: ─ Tissues and cells from most fibrotic diseases not readily accessible as diagnosed late ─ Competitors use animals or late stage cells in culture, neither reflect human disease ─ Our use of human tissue makes preclinical discovery more relevant and accurate, mitigating risk for clinical stage • Currently no competition for targeting and preventing early stage fibrosis • Non - surgical, easy to administer • Short term treatment, intended to halt disease progression Novel Use Of Human Disease Tissue To Identify New Targets In Fibrosis 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
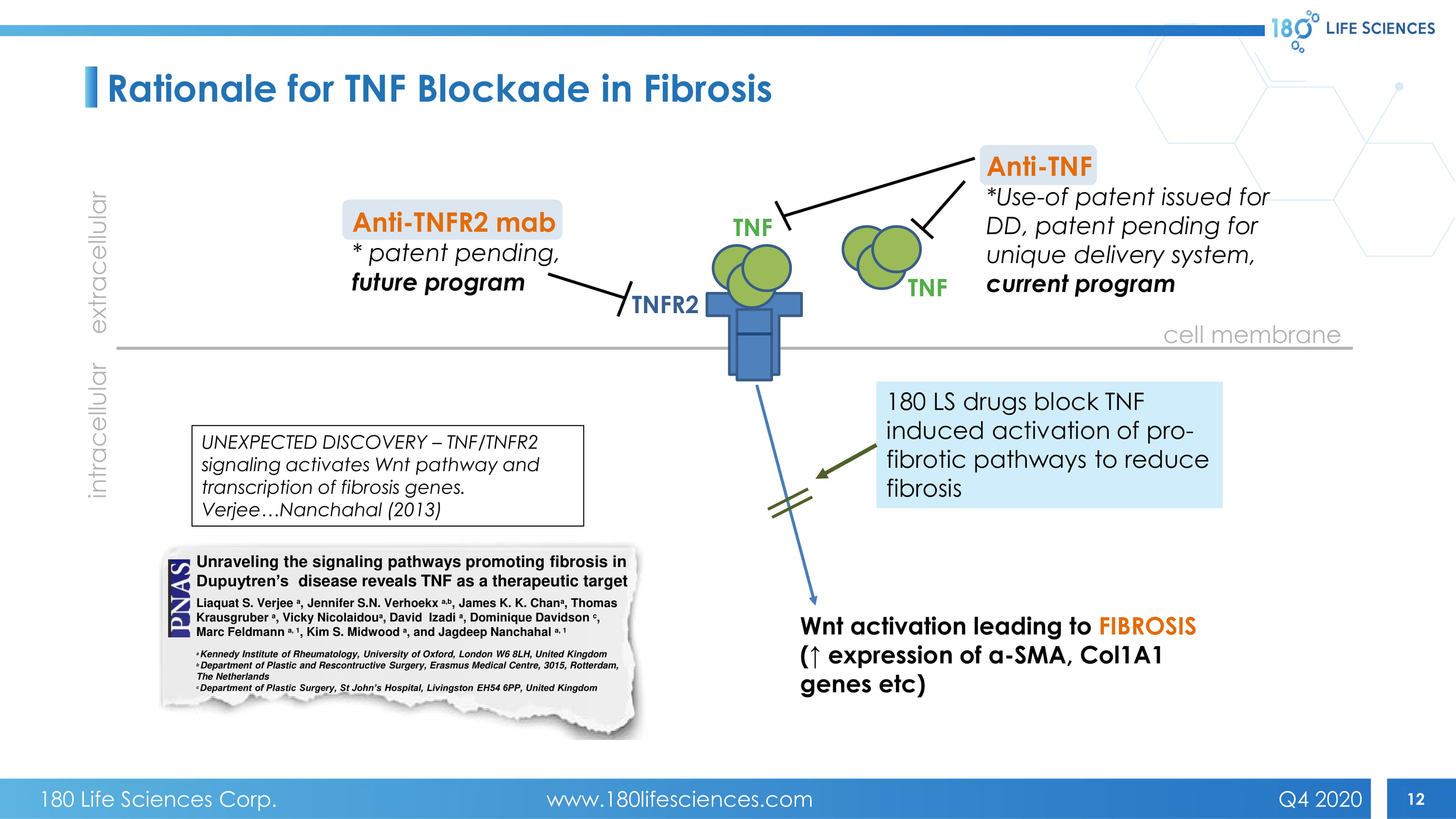
12 TNFR2 cell membrane extracellular intracellular Anti - TNF *Use - of patent issued for DD, patent pending for unique delivery system, current program TNF Anti - TNFR2 mab * patent pending, future program TNF Wnt activation leading to FIBROSIS (↑ expression of a - SMA, Col1A1 genes etc ) UNEXPECTED DISCOVERY – TNF/TNFR2 signaling activates Wnt pathway and transcription of fibrosis genes. Verjee…Nanchahal (2013) 180 LS drugs block TNF induced activation of pro - fibrotic pathways to reduce fibrosis Unraveling the signaling pathways promoting fibrosis in Dupuytren’s disease reveals TNF as a therapeutic target Liaquat S. Verjee a , Jennifer S.N. Verhoekx a,b , James K. K. Chan a , Thomas Krausgruber a , Vicky Nicolaidou a , David Izadi a , Dominique Davidson c , Marc Feldmann a, 1 , Kim S. Midwood a , and Jagdeep Nanchahal a, 1 a Kennedy Institute of Rheumatology, University of Oxford, London W6 8LH, United Kingdom b Department of Plastic and Rescontructive Surgery, Erasmus Medical Centre, 3015, Rotterdam, The Netherlands c Department of Plastic Surgery, St John’s Hospital, Livingston EH54 6PP, United Kingdom Rationale for TNF Blockade in Fibrosis 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
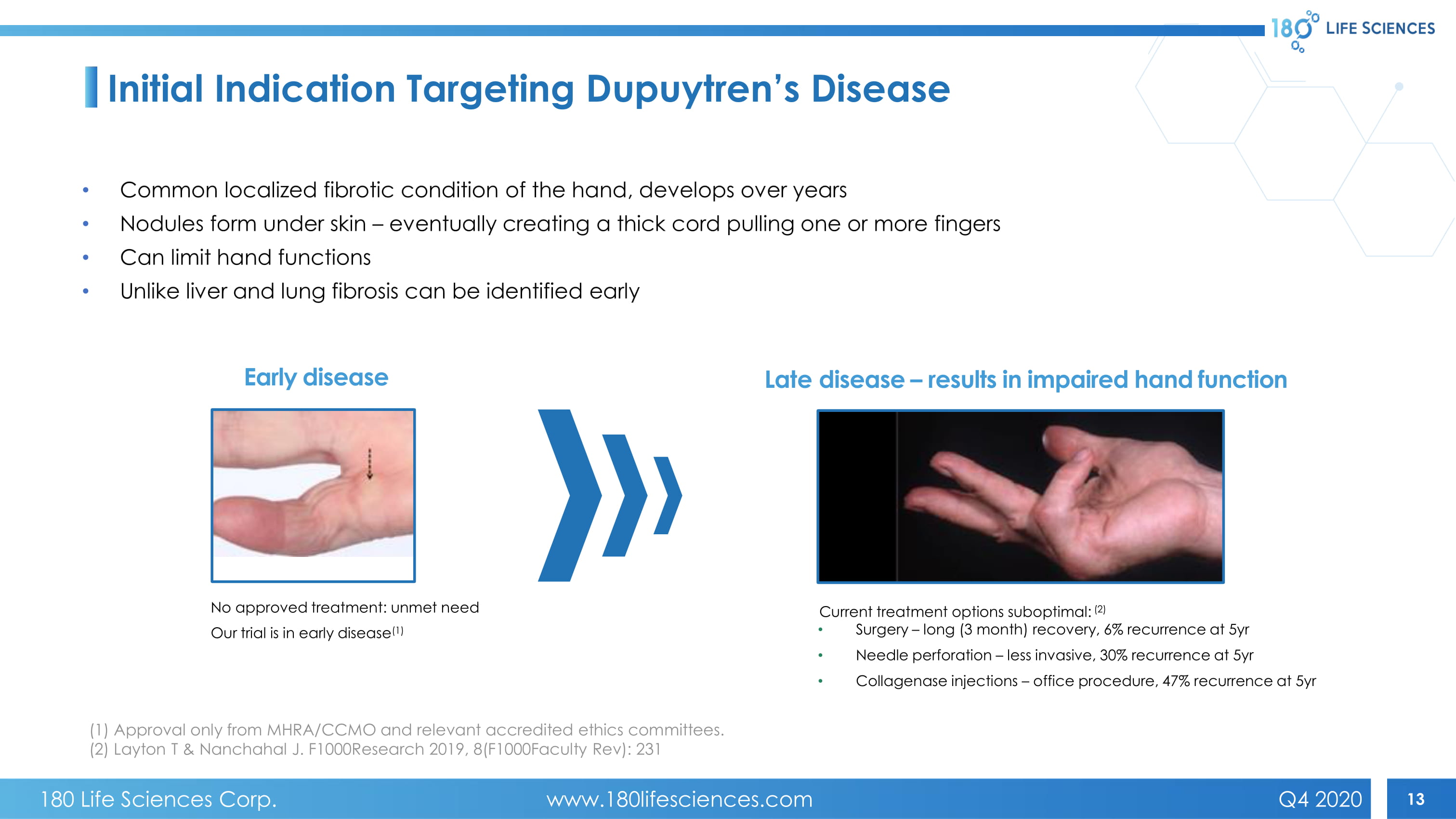
13 • Common localized fibrotic condition of the hand , develops over years • Nodules form under skin – eventually creating a thick cord pulling one or more fingers • Can limit hand functions • Unlike liver and lung fibrosis can be identified early Early disease Late disease – results in impaired hand function No approved treatment : unmet need Our trial is in early disease (1) Current treatment options suboptimal: (2) • Sur gery – long (3 month) recovery, 6% recurrence at 5yr • N eedle perforation – less invasive, 30% recurrence at 5yr • C ollagenase injections – office procedure, 47% recurrence at 5yr Initial Indication Targeting Dupuytren’s Disease (1) Approval only from MHRA/CCMO and relevant accredited ethics committees. (2) Layton T & Nanchahal J. F1000Research 2019, 8(F1000Faculty Rev): 231 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
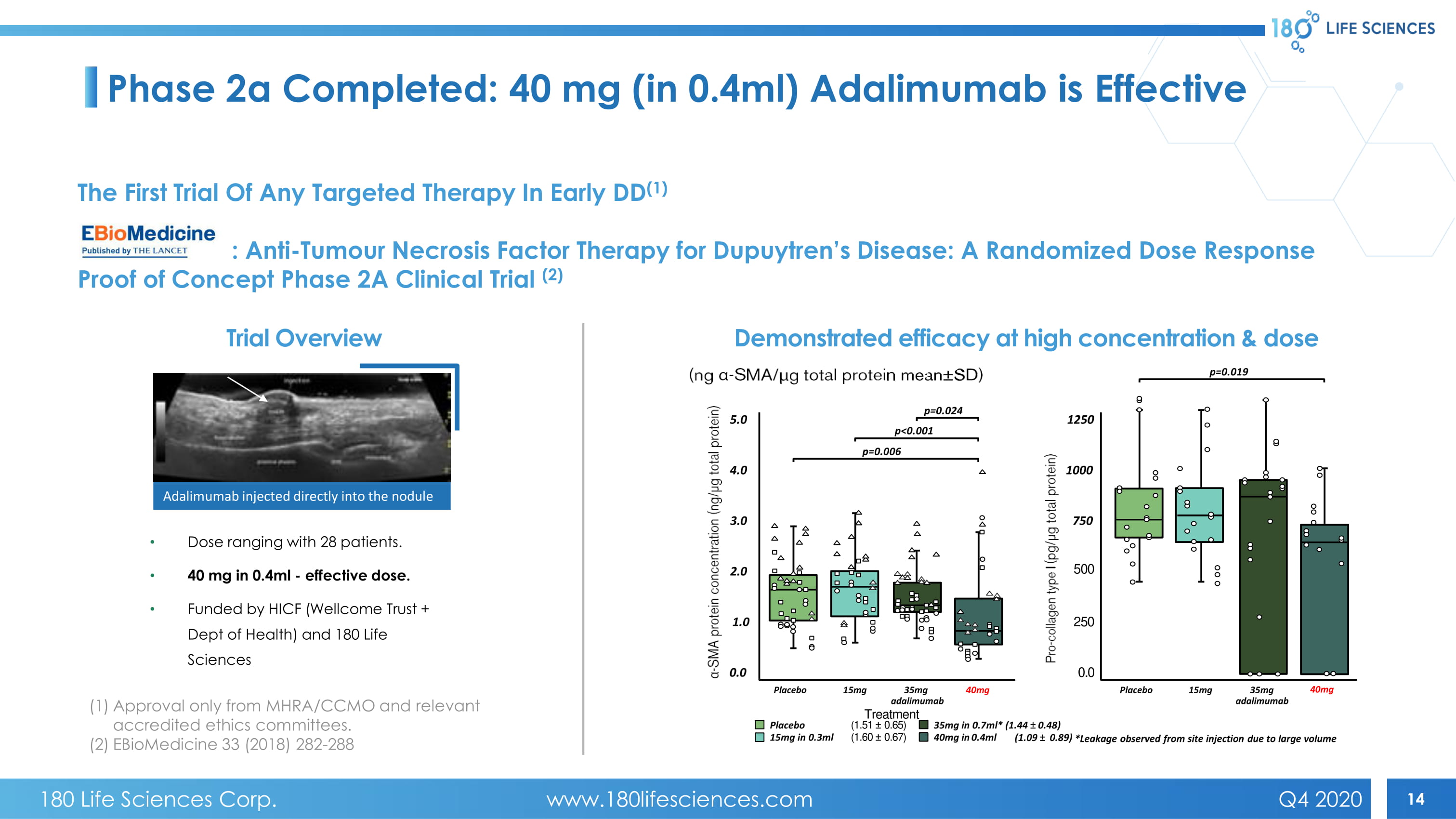
14 Phase 2a Completed: 40 mg (in 0.4ml) Adalimumab is Effective • Dose ranging with 28 patients. • 40 mg in 0.4ml - effective dose. • Funded by HICF ( Wellcome Trust + Dept of Health) and 180 Life Sciences Trial Overview Adalimumab injected directly into the nodule Demonstrated efficacy at high concentration & dose 15mg in 0.3ml (1.60 “ 0.67) 40mg in 0.4ml Placebo (1.51 “ 0.65) 35mg in 0.7ml* (1.44 “ 0.48) (1.09 “ 0.89) *Leakage observed from site injection due to large volume 5.0 4.0 3.0 2.0 1.0 0.0 Placebo 15mg 35mg 40mg a dalimumab Treatment p=0.024 p=0.006 p<0.001 1250 1000 750 500 250 0.0 Placebo 15mg 35mg 40mg a dalimumab p=0.019 The First Trial Of Any Targeted Therapy In Early DD (1) : Anti - Tumour Necrosis Factor Therapy for Dupuytren’s Disease: A Randomized Dose Response Proof of Concept Phase 2A Clinical Trial (2) (1) Approval only from MHRA/CCMO and relevant accredited ethics committees. (2) EBioMedicine 33 (2018) 282 - 288 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
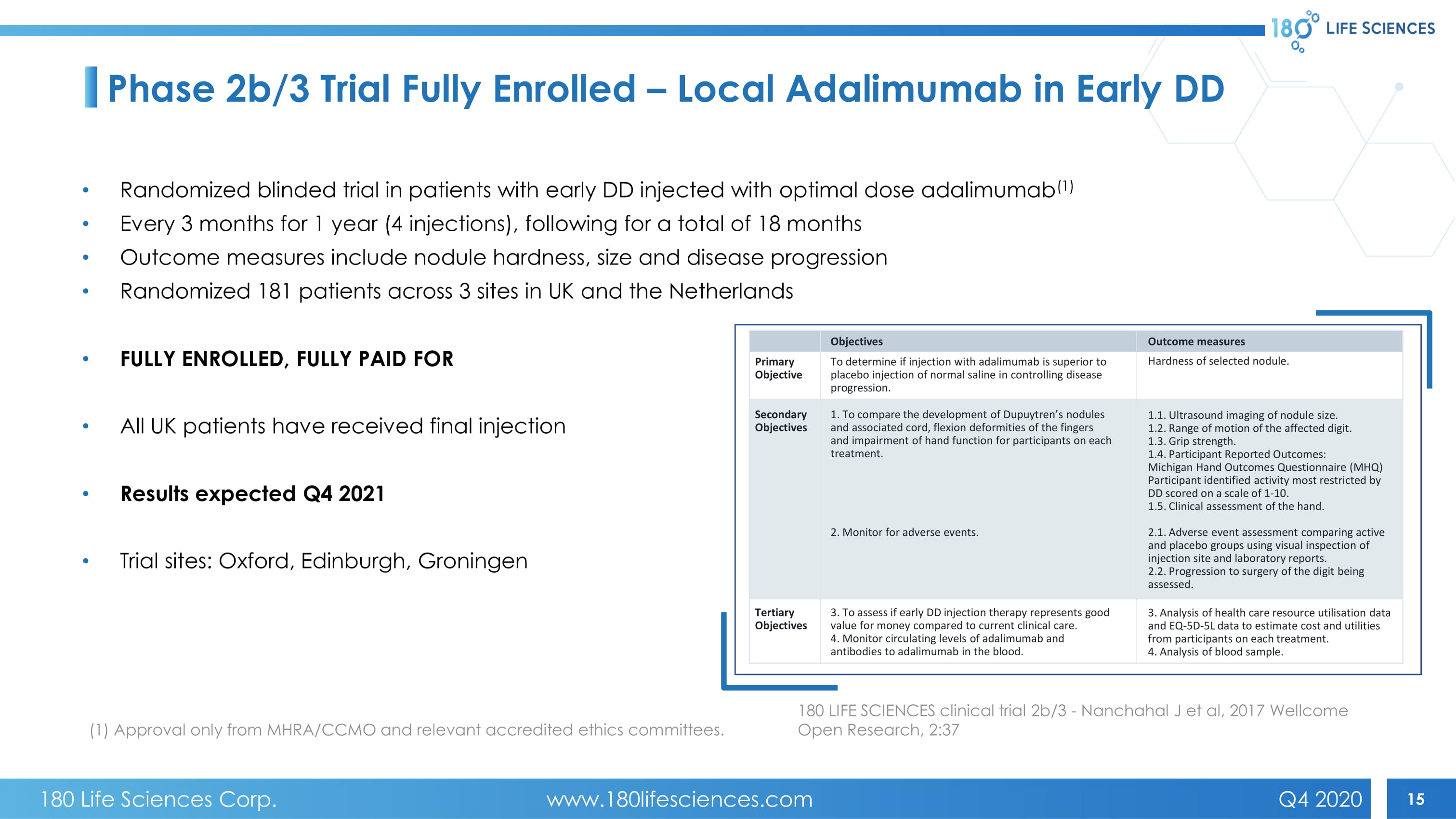
15 180 LIFE SCIENCES clinical trial 2b/3 - Nanchahal J et al, 2017 Wellcome Open Research, 2:37 Phase 2b/3 Trial Fully Enrolled – Local Adalimumab in Early DD • Randomized blinded trial in patients with early DD injected with optimal dose adalimumab (1) • Every 3 months for 1 year (4 injections), following for a total of 18 months • Outcome measures include nodule hardness, size and disease progression • Randomized 181 patients across 3 sites in UK and the Netherlands • FULLY ENROLLED, FULLY PAID FOR • All UK patients have received final injection • Results expected Q4 2021 • Trial sites: Oxford, Edinburgh, Groningen (1) Approval only from MHRA/CCMO and relevant accredited ethics committees. Primary Objective Secondary Objectives Tertiary Objectives To determine if injection with adalimumab is superior to placebo injection of normal saline in controlling disease progression. Hardness of selected nodule. 1. To compare the development of Dupuytren’s nodules and associated cord, flexion deformities of the fingers and impairment of hand function for participants on each treatment. 1.1. Ultrasound imaging of nodule size. 1.2. Range of motion of the affected digit. 1.3. Grip strength. 1.4. Participant Reported Outcomes: Michigan Hand Outcomes Questionnaire (MHQ) Participant identified activity most restricted by DD scored on a scale of 1 - 10. 1.5. Clinical assessment of the hand. 2.1. Adverse event assessment comparing active and placebo groups using visual inspection of injection site and laboratory reports. 2.2. Progression to surgery of the digit being assessed. 3. Analysis of health care resource utilisation data and EQ - 5D - 5L data to estimate cost and utilities from participants on each treatment. 4. Analysis of blood sample. 2. Monitor for adverse events. 3. To assess if early DD injection therapy represents good value for money compared to current clinical care. 4. Monitor circulating levels of adalimumab and antibodies to adalimumab in the blood. Objectives Outcome measures 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
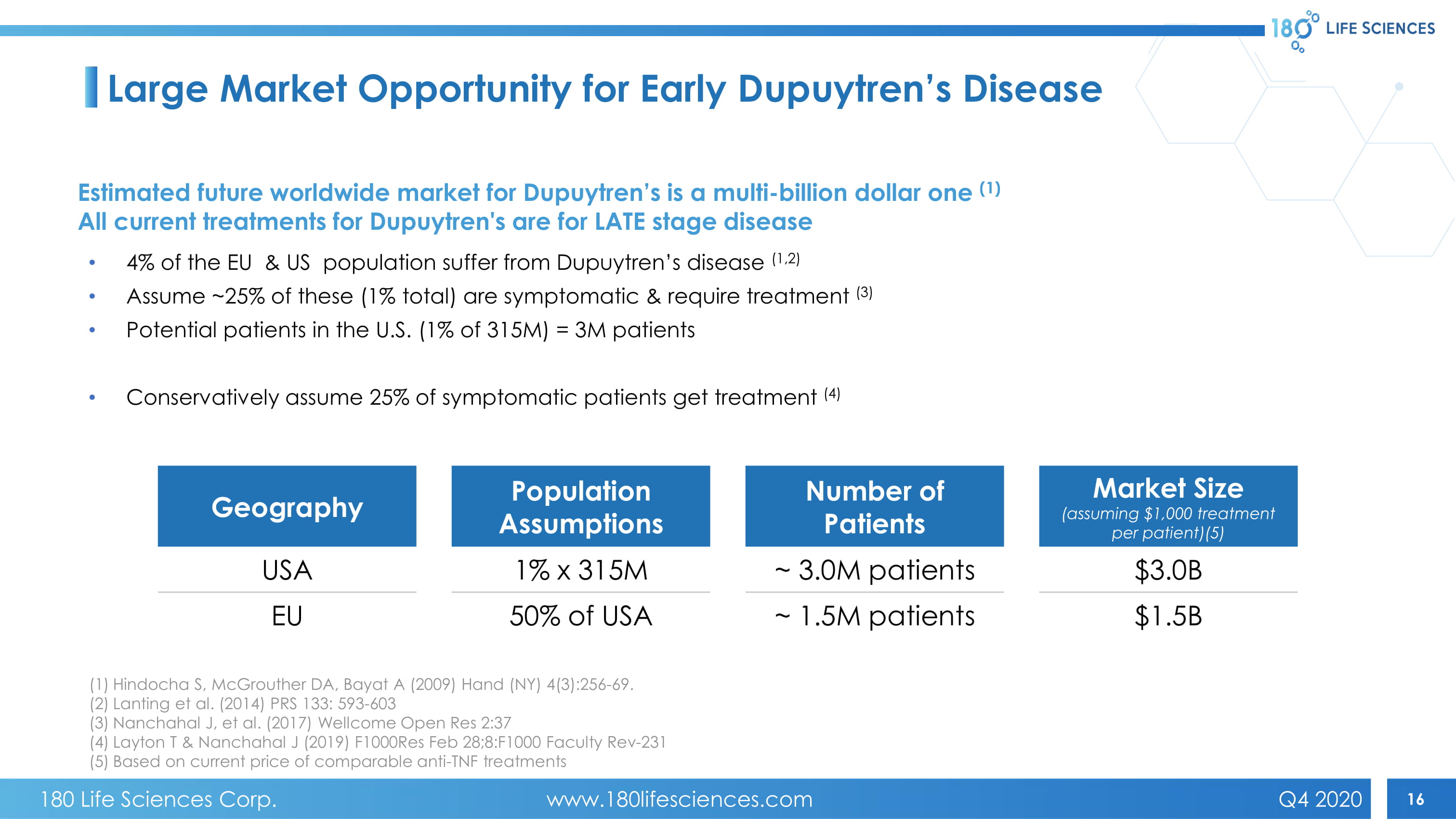
16 Large Market Opportunity for Early Dupuytren’s Disease Estimated future worldwide market for Dupuytren’s is a multi - billion dollar one (1) All current treatments for Dupuytren's are for LATE stage disease • 4% of the EU & US population suffer from Dupuytren’s disease (1,2) • Assume ~25% of these (1% total) are symptomatic & require treatment (3) • Potential patients in the U.S. (1% of 315M) = 3M patients • Conservatively assume 25% of symptomatic patients get treatment (4) Geography Population Assumptions Number of Patients Market Size (assuming $1,000 treatment per patient)(5) USA 1% x 315M ~ 3.0M patients $3.0B EU 50% of USA ~ 1.5M patients $1.5B (1) Hindocha S, McGrouther DA, Bayat A (2009) Hand (NY) 4(3):256 - 69. (2) Lanting et al. (2014) PRS 133: 593 - 603 (3) Nanchahal J, et al. (2017) Wellcome Open Res 2:37 (4) Layton T & Nanchahal J (2019) F1000Res Feb 28;8:F1000 Faculty Rev - 231 (5) Based on current price of comparable anti - TNF treatments 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
17 Additional Indications • Over 300,000 hip fractures each year in the U.S. alone (1) • Strong clinical evidence for anti - TNF as preventative therapy • Patent claims granted, patent is licensed from Kennedy Trust, UK • Phase 2 multi - centre trial of pre - operative anti - TNF in hip fracture surgery planned to initiate by Q4 2021, single dose administered just prior to surgery, to be complete in 4 years Post Operative Delirium/Cognitive Deficit (POCD) Frozen Shoulder • Affects 9% of the of the population aged 25 - 64yr, more common in diabetics (2) • Only treatment for early stage is local steroid injection for short term relief • Phase 2 clinical trials planned for local injection of anti - TNF, initiates Q3 2021 in the UK • Trial protocol completed and £250,000 NIHR grant received (1) https://www.cdc.gov/homeandrecreationalsafety/falls/adulthipfx.html (2) Walker - Bone K et al (2004) Arthritis Rheum 51(4):642 - 651 (3) Rinella ME & Sanyal AJ (2016) Nat Rev Gastroenterol Hepatol 13(4):196 - 205 (4) Ibid. Fibrosis of the Liver (NASH) • Most commonly caused by non - alcoholic fatty liver disease (NAFLD), which affects ~30% of the US population (3) • ~2% of patients with non - alcoholic fatty liver disease and 15 - 20% with non - alcoholic steatohepatitis (NASH) progress to cirrhosis (4) • No approved therapeutic for NASH • Lab program in collaboration with Celgene - BMS for target discovery using human liver samples 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
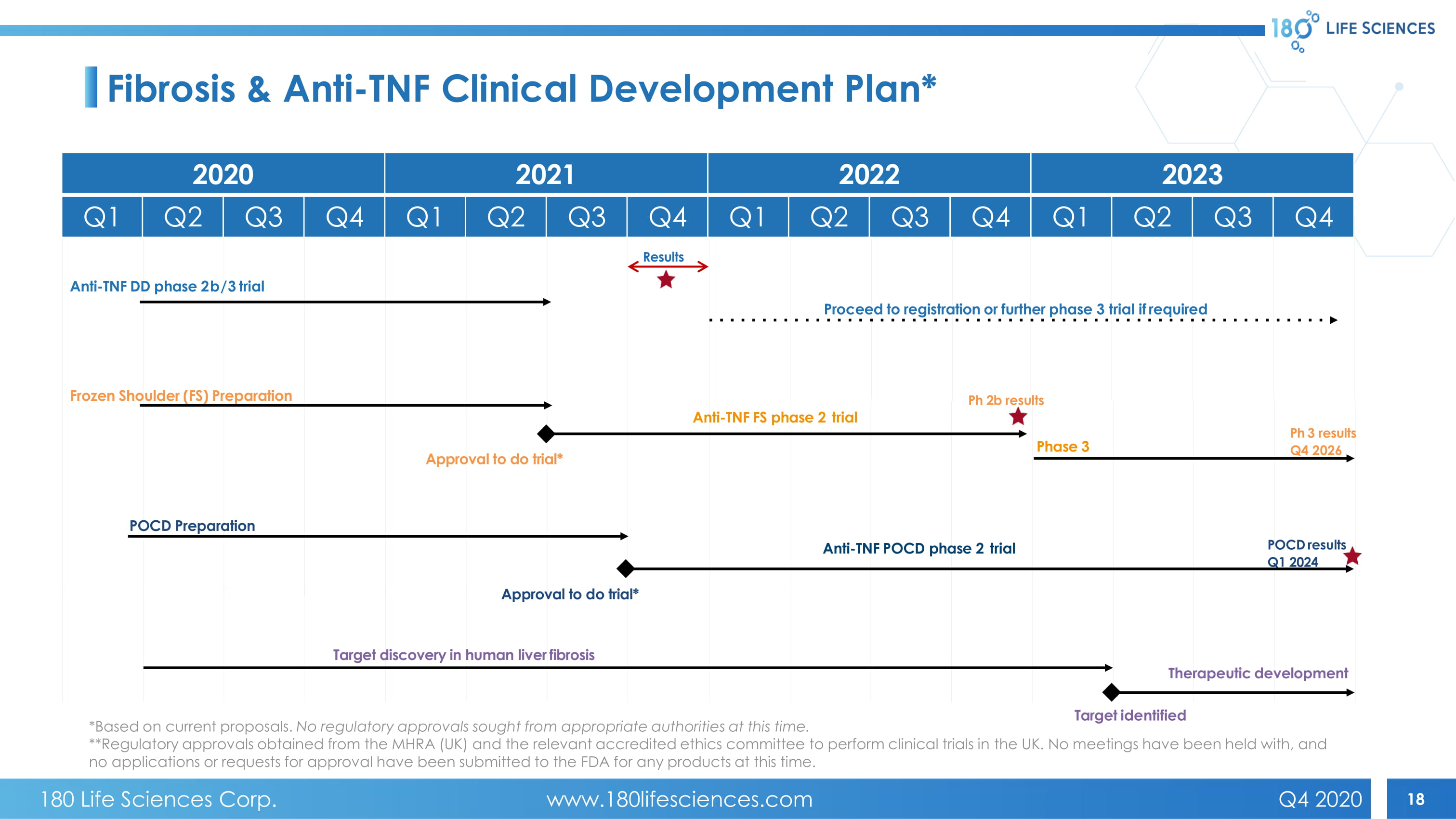
18 Fibrosis & Anti - TNF Clinical Development Plan* Anti - TNF DD phase 2b/3 trial Ph 2b results P h 3 results Q4 2026 POCD results Q1 2024 Frozen Shoulder (FS) Preparation POCD Preparation Approval to do trial* Anti - TNF FS phase 2 trial Therapeutic development Ta rget identified Anti - TNF POCD phase 2 trial Approval to do trial* Phase 3 Results Proceed to registration or further phase 3 trial if required Target discovery in human liver fibrosis 2020 2021 2022 2023 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 *Based on current proposals. No regulatory approvals sought from appropriate authorities at this time. **Regulatory approvals obtained from the MHRA (UK) and the relevant accredited ethics committee to perform clinical trials in th e UK. No meetings have been held with, and no applications or requests for approval have been submitted to the FDA for any products at this time. 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
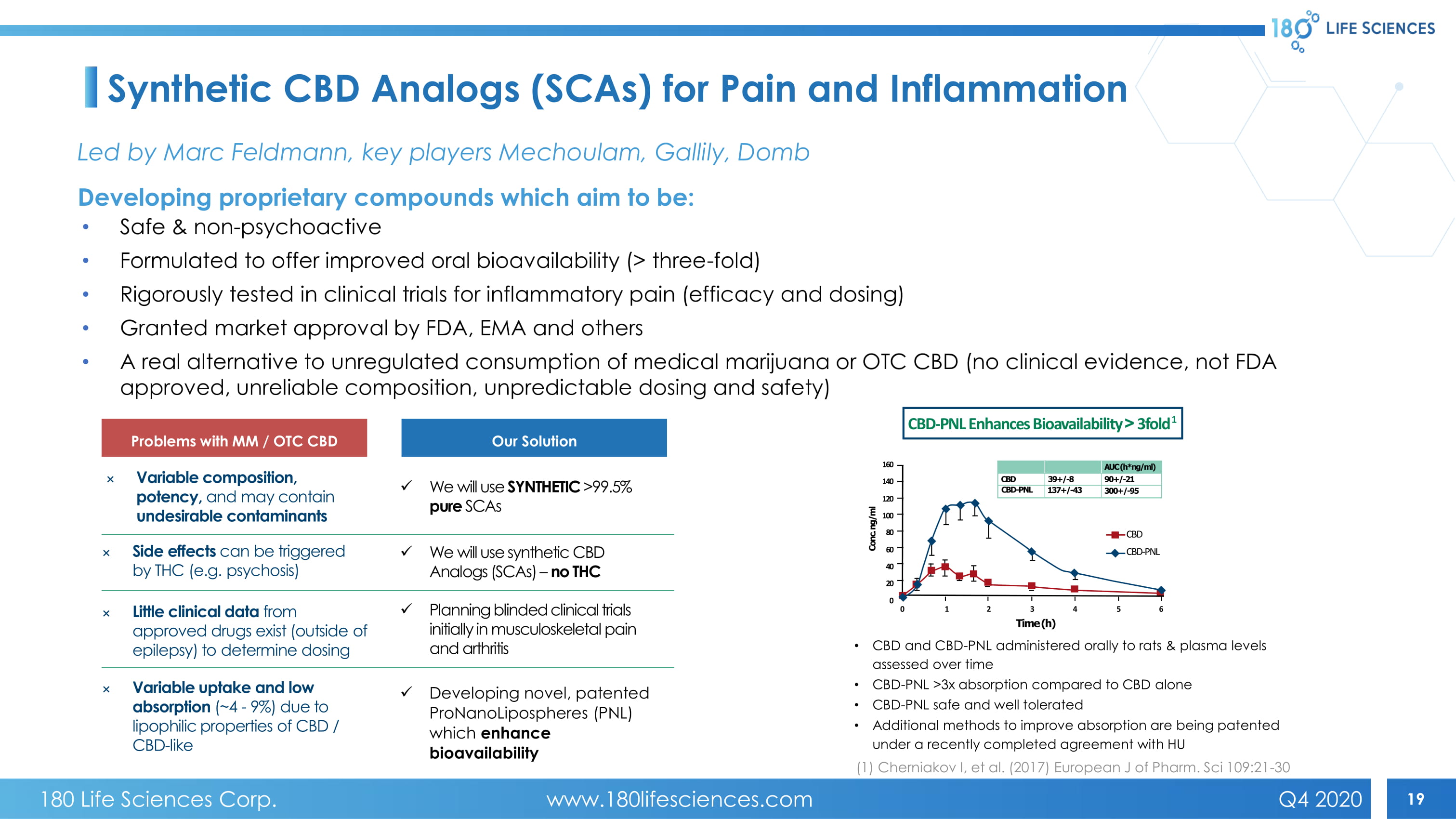
19 Synthetic CBD Analogs (SCAs) for Pain and Inflammation • Safe & non - psychoactive • Formulated to offer improved oral bioavailability (> three - fold) • Rigorously tested in clinical trials for inflammatory pain (efficacy and dosing) • Granted market approval by FDA, EMA and others • A real alternative to unregulated consumption of medical marijuana or OTC CBD (no clinical evidence, not FDA approved, unreliable composition, unpredictable dosing and safety) × Variable composition, potency, and may contain undesirable contaminants x We will use SYNTHETIC >99.5% pure SCAs Problems with MM / OTC CBD Our Solution × Side effects can be triggered by THC (e.g. psychosis) × Little clinical data from approved drugs exist (outside of epilepsy) to determine dosing × Variable uptake and low absorption (~4 - 9%) due to lipophilic properties of CBD / CBD - like x We will use synthetic CBD Analogs (SCAs) – no THC x P lanning blinded clinical trials initially in musculoskeletal pain and arthritis x Developing novel, patented ProNanoLipospheres (PNL) which enhance bioavailability • CBD and CBD - PNL administered orally to rats & plasma l evels assessed over time • CBD - PNL >3x absorption compared to CBD alone • CBD - PNL safe and well tolerated • Additional methods to improve absorption are being patented under a recently completed agreement with HU (1) Cherniakov I, et al. (2017) European J of Pharm. Sci 109:21 - 30 Led by Marc Feldmann, key players Mechoulam , Gallily , Domb Developing proprietary compounds which aim to be: 160 140 120 100 80 60 40 20 0 0 1 2 3 4 5 6 CBD CBD - PNL Time (h) Conc. ng/ml AUC (h*ng/ml) 90+/ - 21 300+/ - 95 39+/ - 8 137+/ - 43 CBD CBD - PNL CBD - PNL Enhances Bioavailability ! 3 fold 1 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
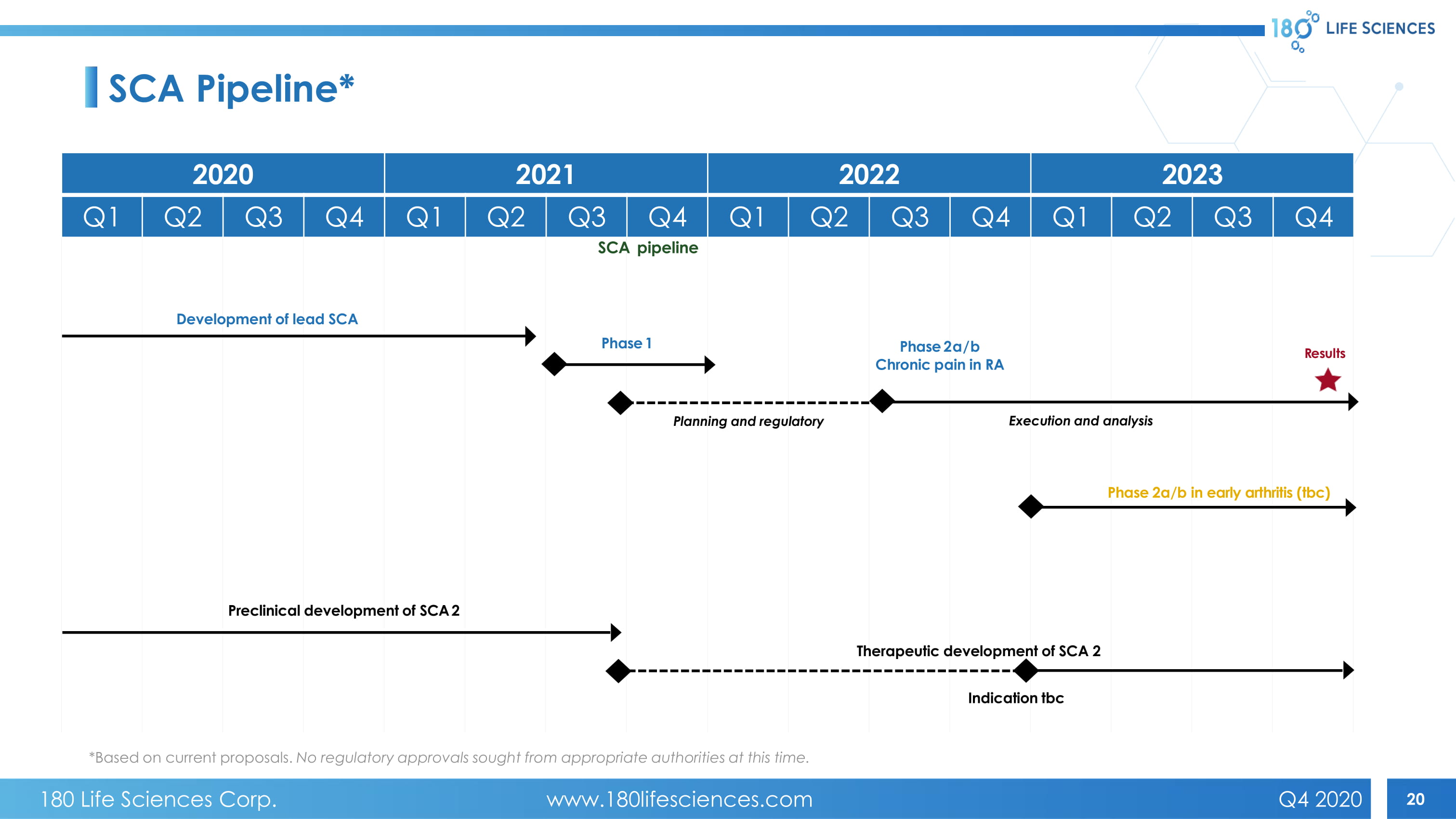
20 SCA Pipeline* Development of l ead SCA Phase 1 Phase 2a/b Chronic pain in RA R e s ul t s SCA pipeline Phase 2a/b in early arthritis (tbc) Therapeutic development of SCA 2 Preclinical development of SCA 2 Indication tbc 2020 2021 2022 2023 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 *Based on current proposals. No regulatory approvals sought from appropriate authorities at this time. Execution and analysis Planning and regulatory 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
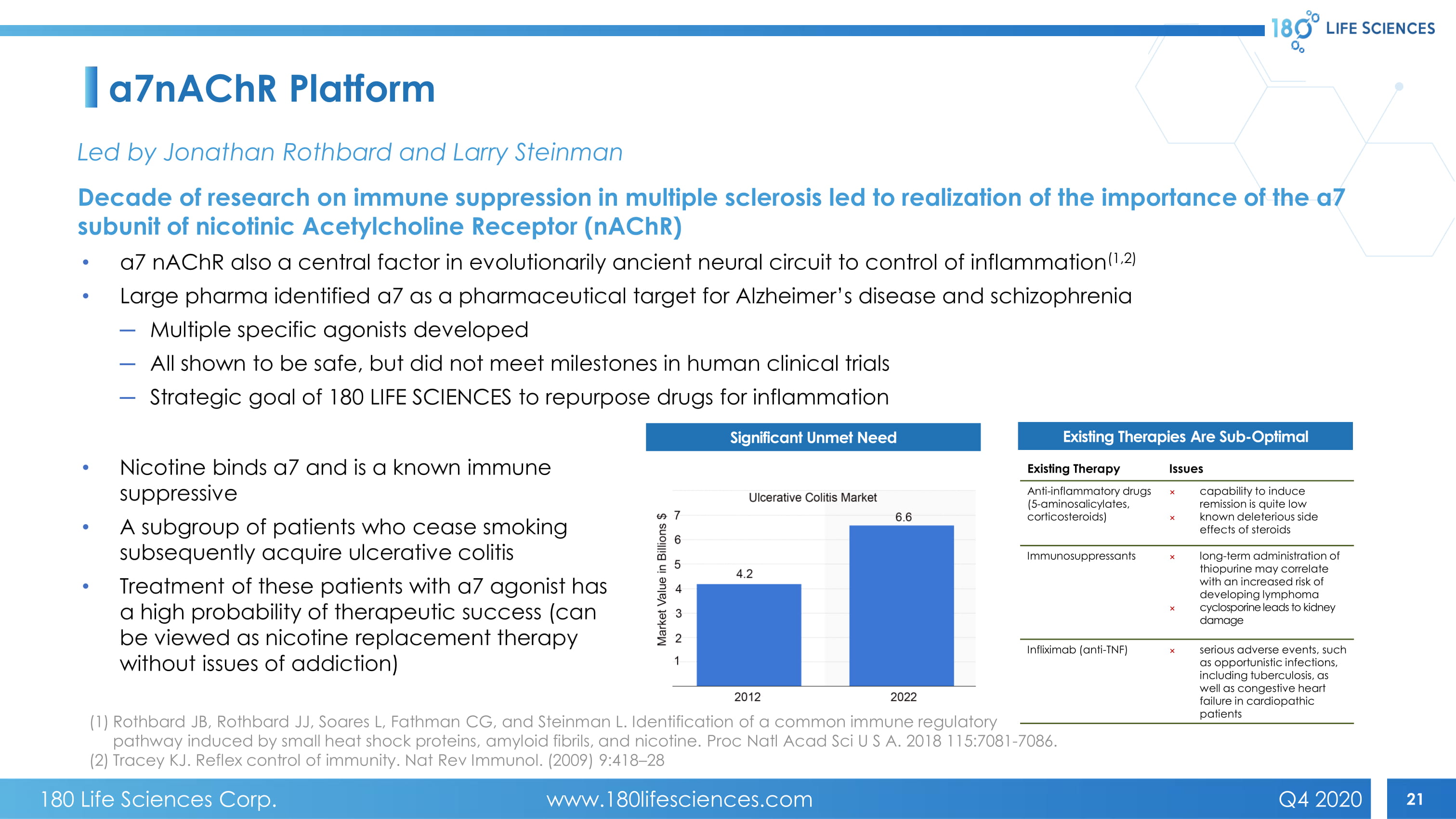
21 a7nAChR Platform Led by Jonathan Rothbard and Larry Steinman Decade of research on immune suppression in multiple sclerosis led to realization of the importance of the a7 subunit of nicotinic Acetylcholine Receptor ( nAChR ) • a 7 nAChR also a central factor in evolutionarily ancient neural circuit to control of inflammation ( 1,2 ) • Large pharma identified a 7 as a pharmaceutical target for Alzheimer’s disease and schizophrenia ─ Multiple specific agonists developed ─ All shown to be safe, but did not meet milestones in human clinical trials ─ Strategic goal of 180 LIFE SCIENCES to repurpose drugs for inflammation (1) Rothbard JB, Rothbard JJ, Soares L, Fathman CG, and Steinman L. Identification of a common immune regulatory pathway induced by small heat shock proteins, amyloid fibrils, and nicotine. Proc Natl Acad Sci U S A. 2018 115:7081 - 7086. (2) Tracey KJ. Reflex control of immunity. Nat Rev Immunol. (2009) 9:418 – 28 Significant Unmet Need Existing Therapies Are Sub - Optimal Existing Therapy Issues Anti - inflammatory drugs (5 - aminosalicylates, corticosteroids) × capability to induce remission is quite low × known deleterious side effects of steroids Immunosuppressants × long - term administration of thiopurine may correlate with an increased risk of developing lymphoma × cyclosporine leads to kidney damage Infliximab (anti - TNF) × serious adverse events, such as opportunistic infections, including tuberculosis, as well as congestive heart failure in cardiopathic patients • Nicotine binds a7 and is a known immune suppressive • A subgroup of patients who cease smoking subsequently acquire ulcerative colitis • Treatment of these patients with a7 agonist has a high probability of therapeutic success (can be viewed as nicotine replacement therapy without issues of addiction) 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
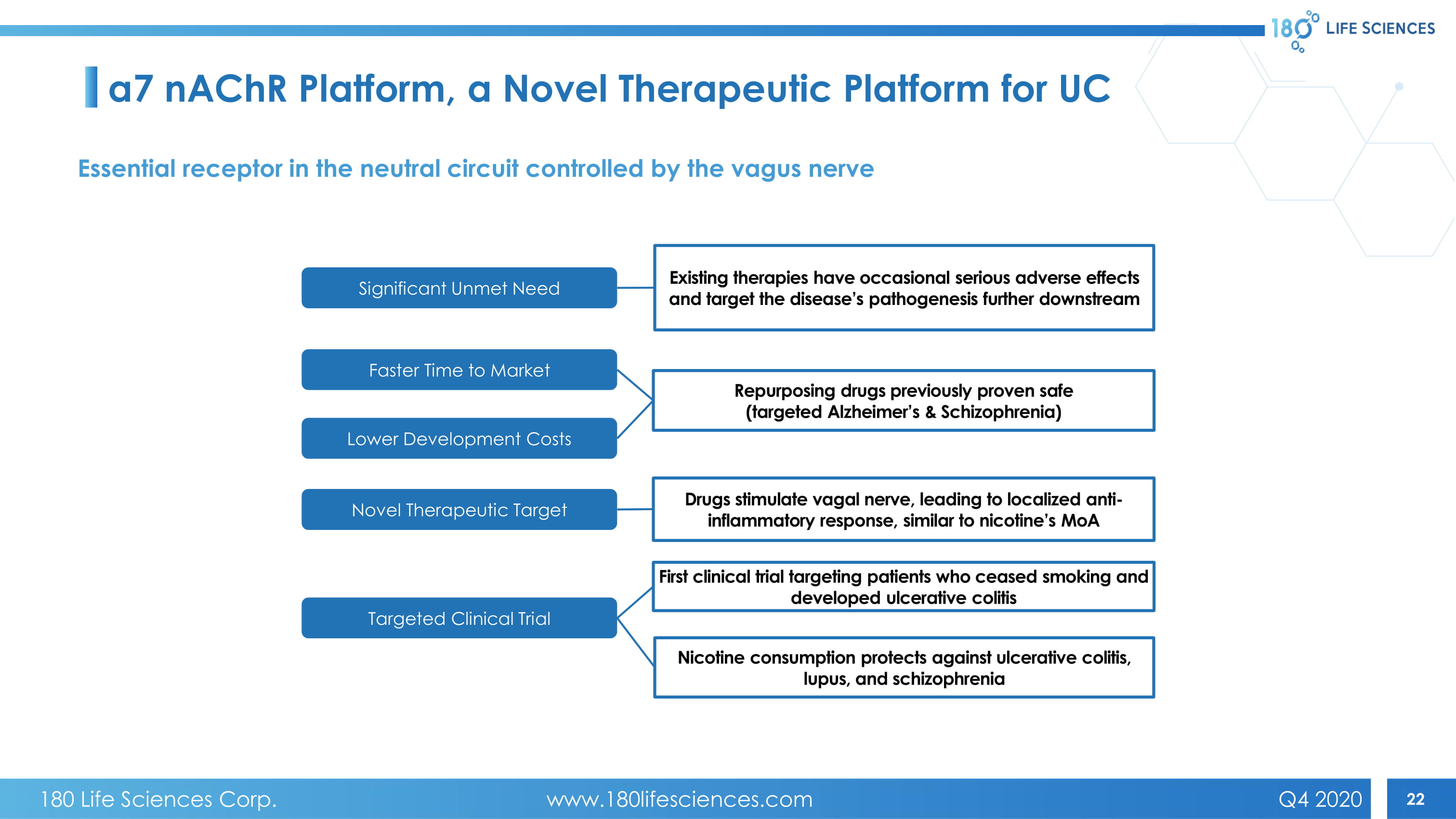
22 Faster Time to Market Lower Development Costs Targeted Clinical Trial Novel Therapeutic Target Significant Unmet Need Repurposing drugs previously proven safe (targeted Alzheimer’s & Schizophrenia) Drugs stimulate vagal nerve, leading to localized anti - inflammatory response, similar to nicotine’s MoA Existing therapies have occasional serious adverse effects and target the disease’s pathogenesis further downstream First clinical trial targeting patients who ceased smoking and developed ulcerative colitis Nicotine consumption protects against ulcerative colitis, lupus, and schizophrenia a7 nAChR Platform, a Novel Therapeutic Platform for UC Essential receptor in the neutral circuit controlled by the vagus nerve 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
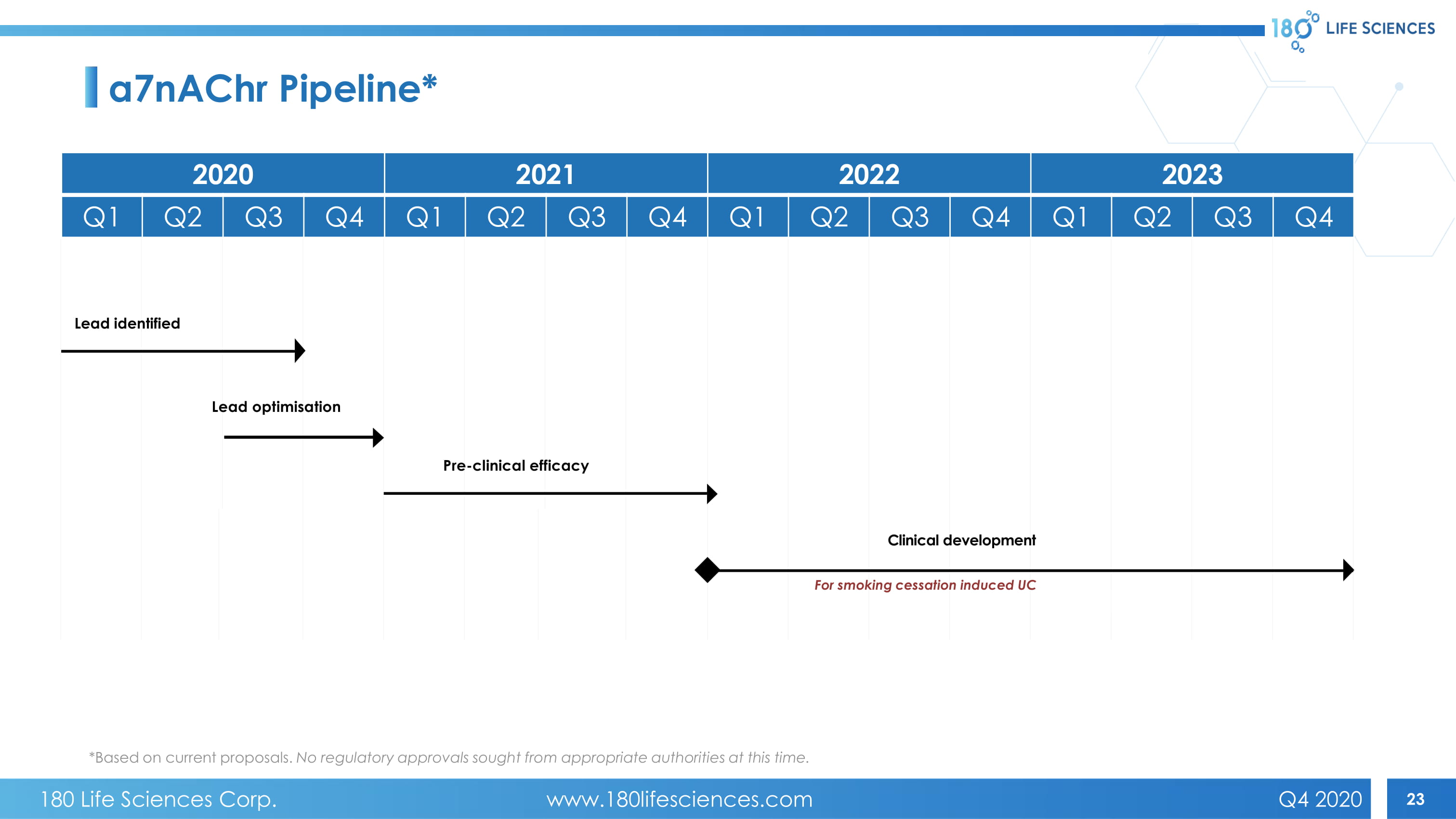
23 Pre - clinical efficacy Lead identified Lead optimisation Clinical development For smoking cessation induced UC a7nAChr Pipeline* 2020 2021 2022 2023 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 *Based on current proposals. No regulatory approvals sought from appropriate authorities at this time. 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
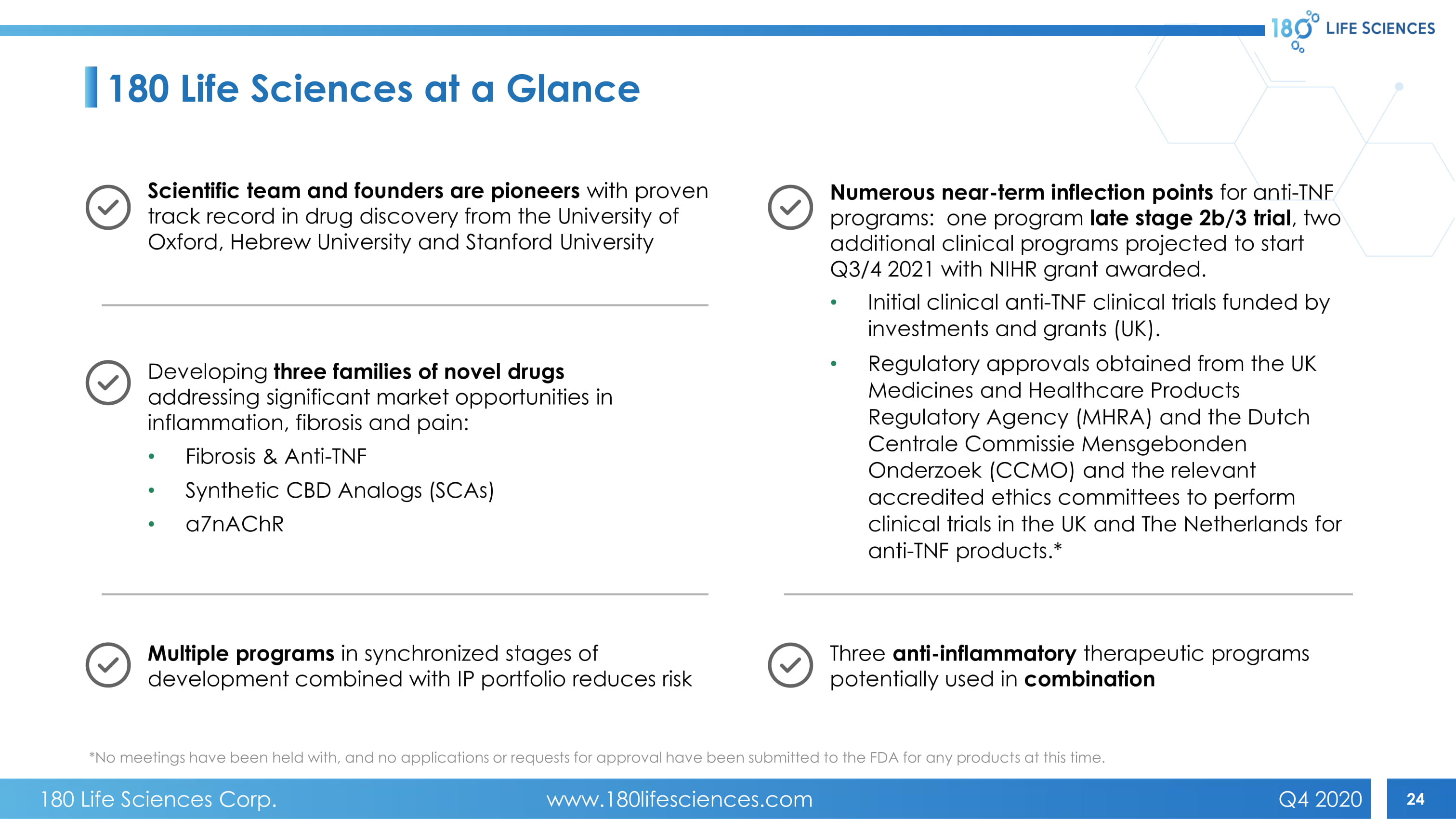
24 180 Life Sciences at a Glance *No meetings have been held with, and no applications or requests for approval have been submitted to the FDA for any product s a t this time. Scientific team and founders are pioneers with proven track record in drug discovery from the University of Oxford, Hebrew University and Stanford University Developing three families of novel drugs addressing significant market opportunities in inflammation, fibrosis and pain: • Fibrosis & Anti - TNF • Synthetic CBD Analogs (SCAs) • a7nAChR Multiple programs in synchronized stages of development combined with IP portfolio reduces risk Numerous near - term inflection points for anti - TNF programs: one program late stage 2b/3 trial , two additional clinical programs projected to start Q3/4 2021 with NIHR grant awarded. • Initial clinical anti - TNF clinical trials funded by investments and grants (UK). • Regulatory approvals obtained from the UK Medicines and Healthcare Products Regulatory Agency (MHRA) and the Dutch Centrale Commissie Mensgebonden Onderzoek (CCMO) and the relevant accredited ethics committees to perform clinical trials in the UK and The Netherlands for anti - TNF products.* Three anti - inflammatory therapeutic programs potentially used in combination 180 Life Sciences Corp. www.180lifesciences.com Q4 2020

www.180lifesciences.com 830 Menlo Avenue, Suite 100, Menlo Park, CA 94025 Thank you 25

Appendix 26
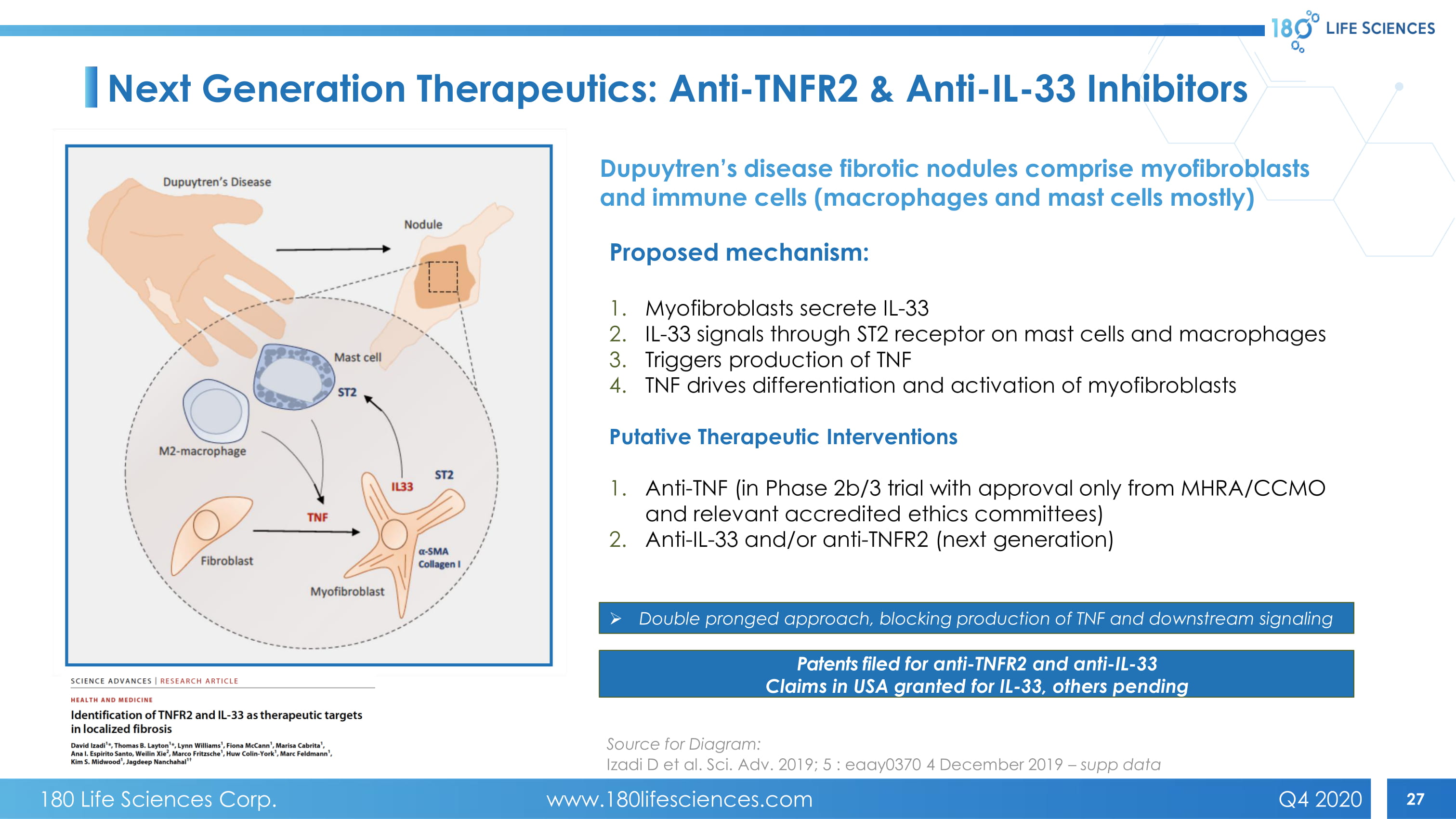
27 Next Generation Therapeutics: Anti - TNFR2 & Anti - IL - 33 Inhibitors Proposed mechanism: 1. Myofibroblasts secrete IL - 33 2. IL - 33 signals through ST2 receptor on mast cells and macrophages 3. Triggers production of TNF 4. TNF drives differentiation and activation of myofibroblasts Putative Therapeutic Interventions 1. Anti - TNF (in Phase 2b/3 trial with approval only from MHRA/CCMO and relevant accredited ethics committees) 2. Anti - IL - 33 and/or anti - TNFR2 (next generation) » Double pronged approach, blocking production of TNF and downstream signaling Patents filed for anti - TNFR2 and anti - IL - 33 Claims in USA granted for IL - 33, others pending Dupuytren’s disease fibrotic nodules comprise myofibroblasts and immune cells (macrophages and mast cells mostly) Source for Diagram: Izadi D et al. Sci. Adv. 2019; 5 : eaay0370 4 December 2019 – supp data 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
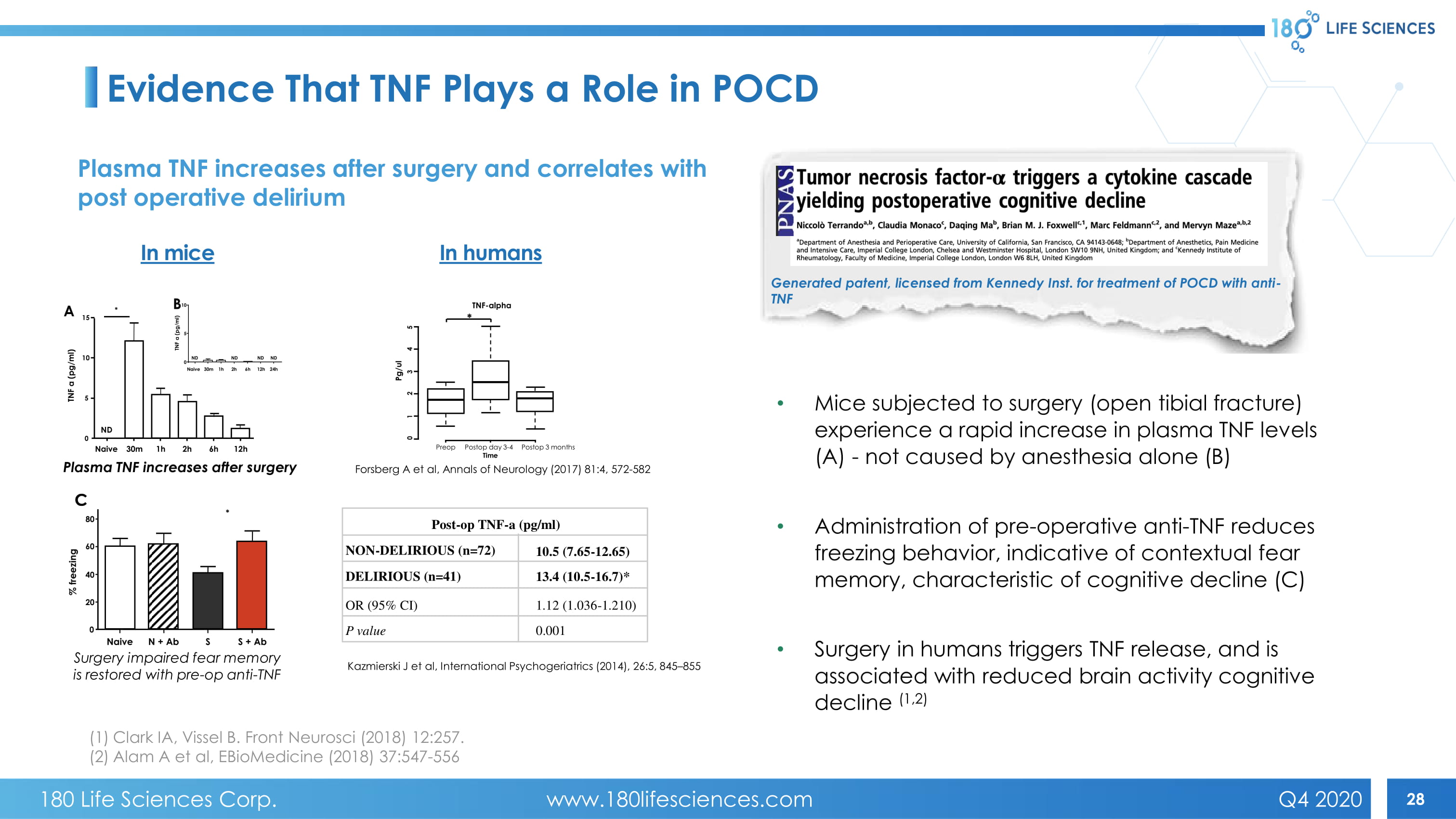
28 C Plasma TNF increases after surgery Surgery impaired fear memory is restored with pre - op anti - TNF Generated patent, licensed from Kennedy Inst. for treatment of POCD with anti - TNF Evidence That TNF Plays a Role in POCD In mice Forsberg A et al, Annals of Neurology (2017) 81:4, 572 - 582 Kazmierski J et al, International Psychogeriatrics (2014), 26:5, 845 – 855 In humans • Mice subjected to surgery (open tibial fracture) experience a rapid increase in plasma TNF levels (A) - not caused by anesthesia alone (B) • Administration of pre - operative anti - TNF reduces freezing behavior, indicative of contextual fear memory, characteristic of cognitive decline (C) • Surgery in humans triggers TNF release, and is associated with reduced brain activity cognitive decline (1,2) Plasma TNF increases after surgery and correlates with post operative delirium (1) Clark IA, Vissel B. Front Neurosci (2018) 12:257. (2) Alam A et al, EBioMedicine (2018) 37:547 - 556 Post - op TNF - a (pg/ml) NON - DELIRIOUS (n=72) DELIRIOUS (n=41) OR (95% CI) P value 10.5 (7.65 - 12.65) 13.4 (10.5 - 16.7)* 1.12 (1.036 - 1.210) 0.001 TNF - alpha Pg/ul Preop Postop day 3 - 4 Time Postop 3 months * 5 4 3 2 1 0 * A 15 10 5 0 Naive 30m 1h 2h 6h 12h TNF a (pg/ml) ND B TNF a (pg/ml) Naive 30m 1h 2h 6h 12h 24h 10 5 0 ND ND ND ND * 80 60 40 20 0 % freezing C Naive N + Ab S S + Ab 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
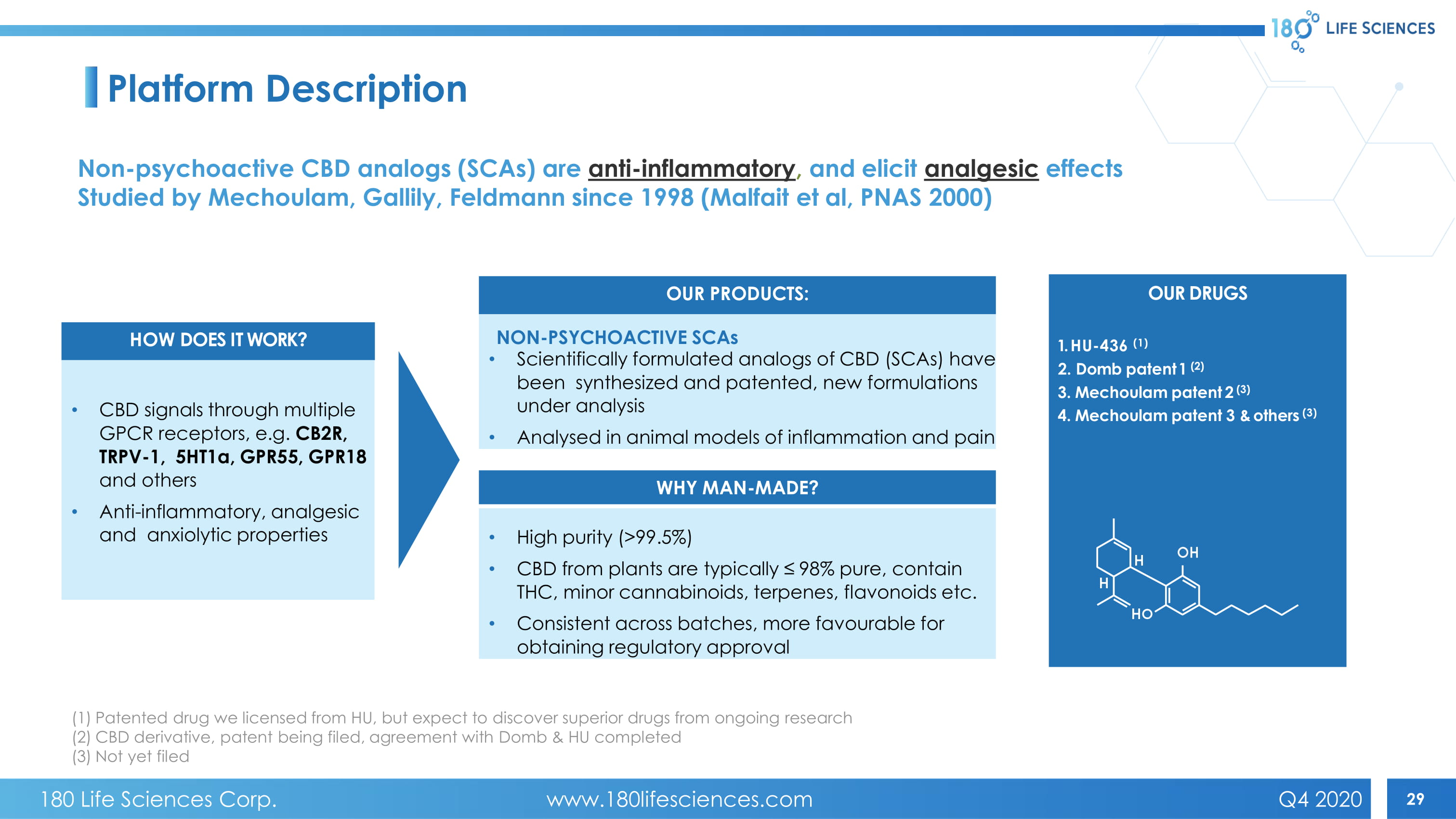
29 Platform Description OUR DRUGS 1. HU - 436 (1) 2. Domb patent 1 (2) 3. Mechoulam patent 2 (3) 4. Mechoulam patent 3 & others (3) HOW DOES IT WORK? OUR PRODUCTS: WHY MAN - MADE? • CBD signals through multiple GPCR receptors, e.g. CB2R, TRPV - 1, 5HT1α, GPR55, GPR18 and others • Anti - inflammatory, analgesic and anxiolytic properties NON - PSYCHOACTIVE SCAs • Scientifically formulated analogs of CBD (SCAs) have been synthesized and patented , new formulations under analysis • Analysed in animal models of inflammation and pain • High purity (>99.5%) • CBD from plants are typically ≤ 98% pure, contain THC, minor cannabinoids, terpenes, flavonoids etc. • Consistent across batches , more favourable for obtaining regulatory approval Non - psychoactive CBD analogs (SCAs) are anti - inflammatory , and elicit analgesic effects Studied by Mechoulam , Gallily , Feldmann since 1998 (Malfait et al, PNAS 2000) (1) Patented drug we licensed from HU, but expect to discover superior drugs from ongoing research (2) CBD derivative, patent being filed, agreement with Domb & HU completed (3) Not yet filed 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
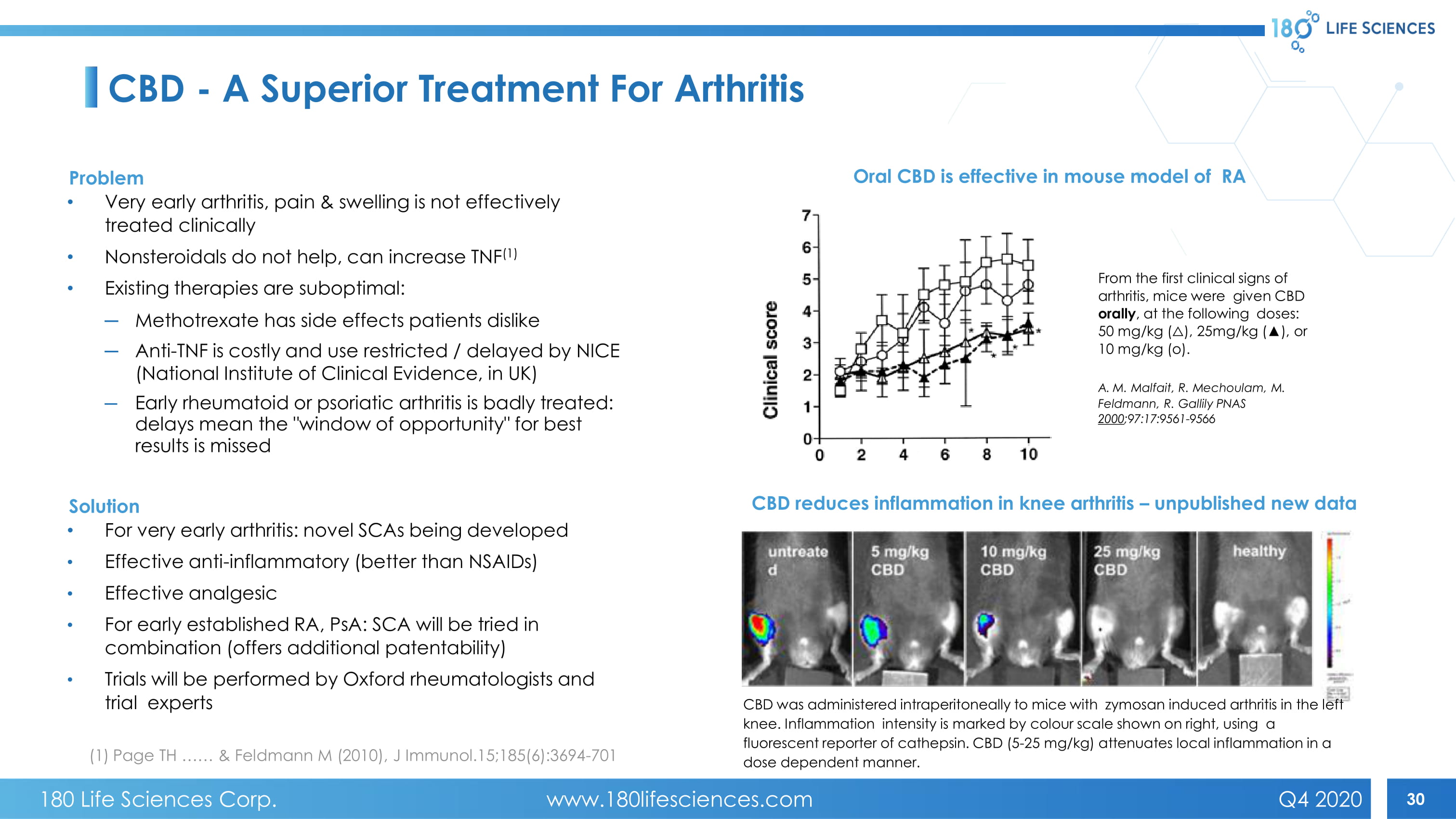
30 CBD - A Superior Treatment For Arthritis Problem • Very early arthritis, pain & swelling is not effectively treated clinically • Nonsteroidals do not help, can increase TNF (1) • Existing therapies are suboptimal: ─ Methotrexate has side effects patients dislike ─ Anti - TNF is costly and use restricted / delayed by NICE (National Institute of Clinical Evidence, in UK) ─ Early rheumatoid or psoriatic arthritis is badly treated: delays mean the " window of opportunity " for best results is missed Solution • For v ery early arthritis: novel SCAs being developed • Effective anti - inflammatory (better than NSAIDs) • Effective analgesic • For e arly established RA, PsA : SCA will be tried in combination (offers additional patentability) • Trials will be performed by Oxford rheumatologists and trial experts Oral CBD is effective in mouse model of RA From the first clinical signs of arthritis, mice were given CBD orally , at the following doses: 50 mg/kg ( ᇞ ), 25mg/kg ( Ÿ ), or 10 mg/kg (o). A. M. Malfait, R. Mechoulam , M. Feldmann, R. Gallily PNAS 2000 ;97:17:9561 - 9566 CBD reduces inflammation in knee arthritis – unpublished new data CBD was administered intraperitoneally to mice with zymosan induced arthritis in the left knee. Inflammation intensity is marked by colour scale shown on right, using a fluorescent reporter of cathepsin. CBD (5 - 25 mg/kg) attenuates local inflammation in a dose dependent manner. (1) Page TH …… & Feldmann M (2010), J Immunol.15;185(6):3694 - 701 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
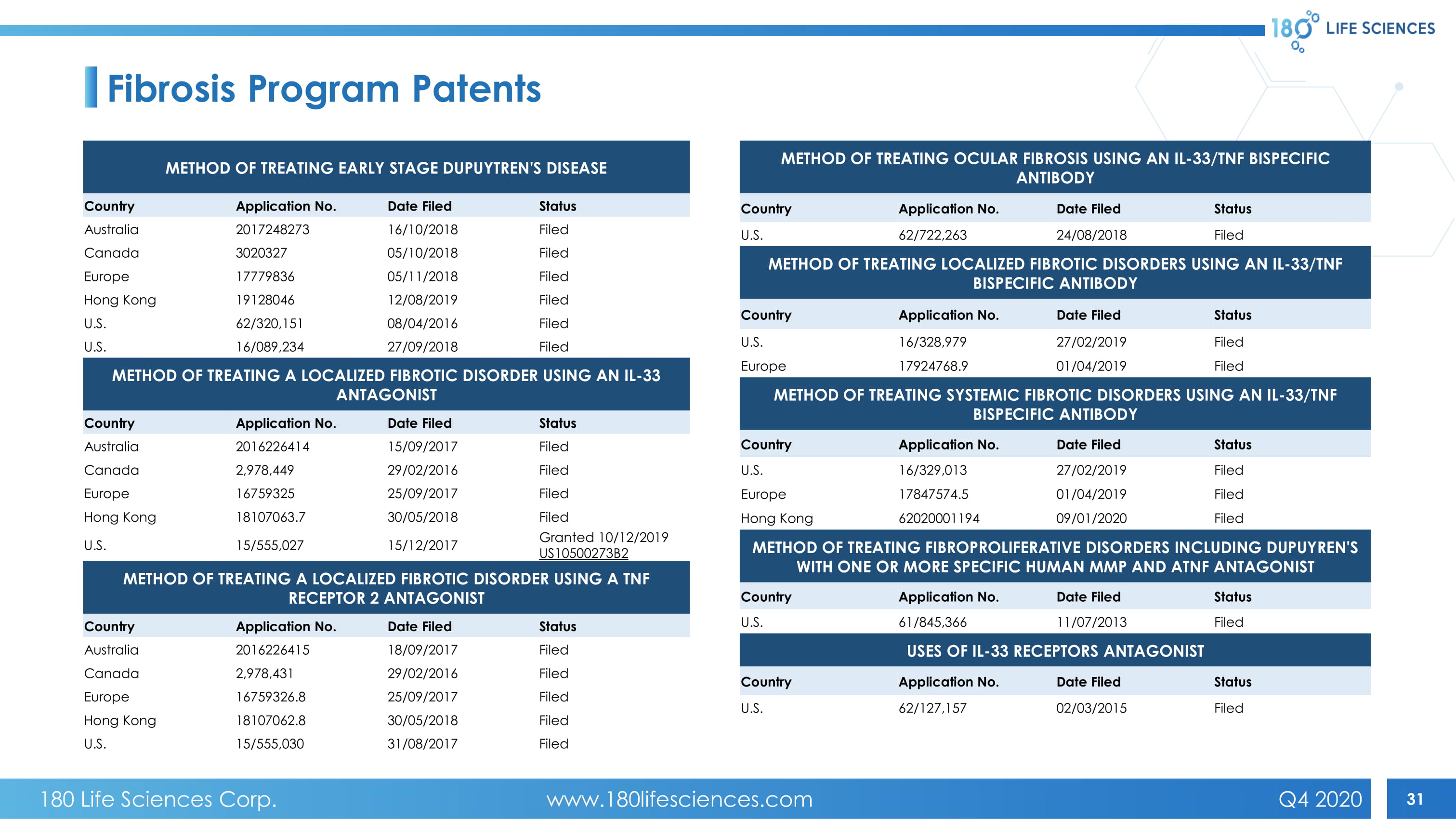
31 Fibrosis Program Patents METHOD OF TREATING EARLY STAGE DUPUYTREN'S DISEASE Country Application No. Date Filed Status Australia 2017248273 16/10/2018 Filed Canada 3020327 05/10/2018 Filed Europe 17779836 05/11/2018 Filed Hong Kong 19128046 12/08/2019 Filed U.S. 62/320,151 08/04/2016 Filed U.S. 16/089,234 27/09/2018 Filed METHOD OF TREATING A LOCALIZED FIBROTIC DISORDER USING AN IL - 33 ANTAGONIST Country Application No. Date Filed Status Australia 2016226414 15/09/2017 Filed Canada 2,978,449 29/02/2016 Filed Europe 16759325 25/09/2017 Filed Hong Kong 18107063.7 30/05/2018 Filed U.S. 15/555,027 15/12/2017 Granted 10/12/2019 US10500273B2 METHOD OF TREATING A LOCALIZED FIBROTIC DISORDER USING A TNF RECEPTOR 2 ANTAGONIST Country Application No. Date Filed Status Australia 2016226415 18/09/2017 Filed Canada 2,978,431 29/02/2016 Filed Europe 16759326.8 25/09/2017 Filed Hong Kong 18107062.8 30/05/2018 Filed U.S. 15/555,030 31/08/2017 Filed METHOD OF TREATING OCULAR FIBROSIS USING AN IL - 33/TNF BISPECIFIC ANTIBODY Country Application No. Date Filed Status U.S. 62/722,263 24/08/2018 Filed METHOD OF TREATING LOCALIZED FIBROTIC DISORDERS USING AN IL - 33/TNF BISPECIFIC ANTIBODY Country Application No. Date Filed Status U.S. 16/328,979 27/02/2019 Filed Europe 17924768.9 01/04/2019 Filed METHOD OF TREATING SYSTEMIC FIBROTIC DISORDERS USING AN IL - 33/TNF BISPECIFIC ANTIBODY Country Application No. Date Filed Status U.S. 16/329,013 27/02/2019 Filed Europe 17847574.5 01/04/2019 Filed Hong Kong 62020001194 09/01/2020 Filed METHOD OF TREATING FIBROPROLIFERATIVE DISORDERS INCLUDING DUPUYREN'S WITH ONE OR MORE SPECIFIC HUMAN MMP AND ATNF ANTAGONIST Country Application No. Date Filed Status U.S. 61/845,366 11/07/2013 Filed USES OF IL - 33 RECEPTORS ANTAGONIST Country Application No. Date Filed Status U.S. 62/127,157 02/03/2015 Filed 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
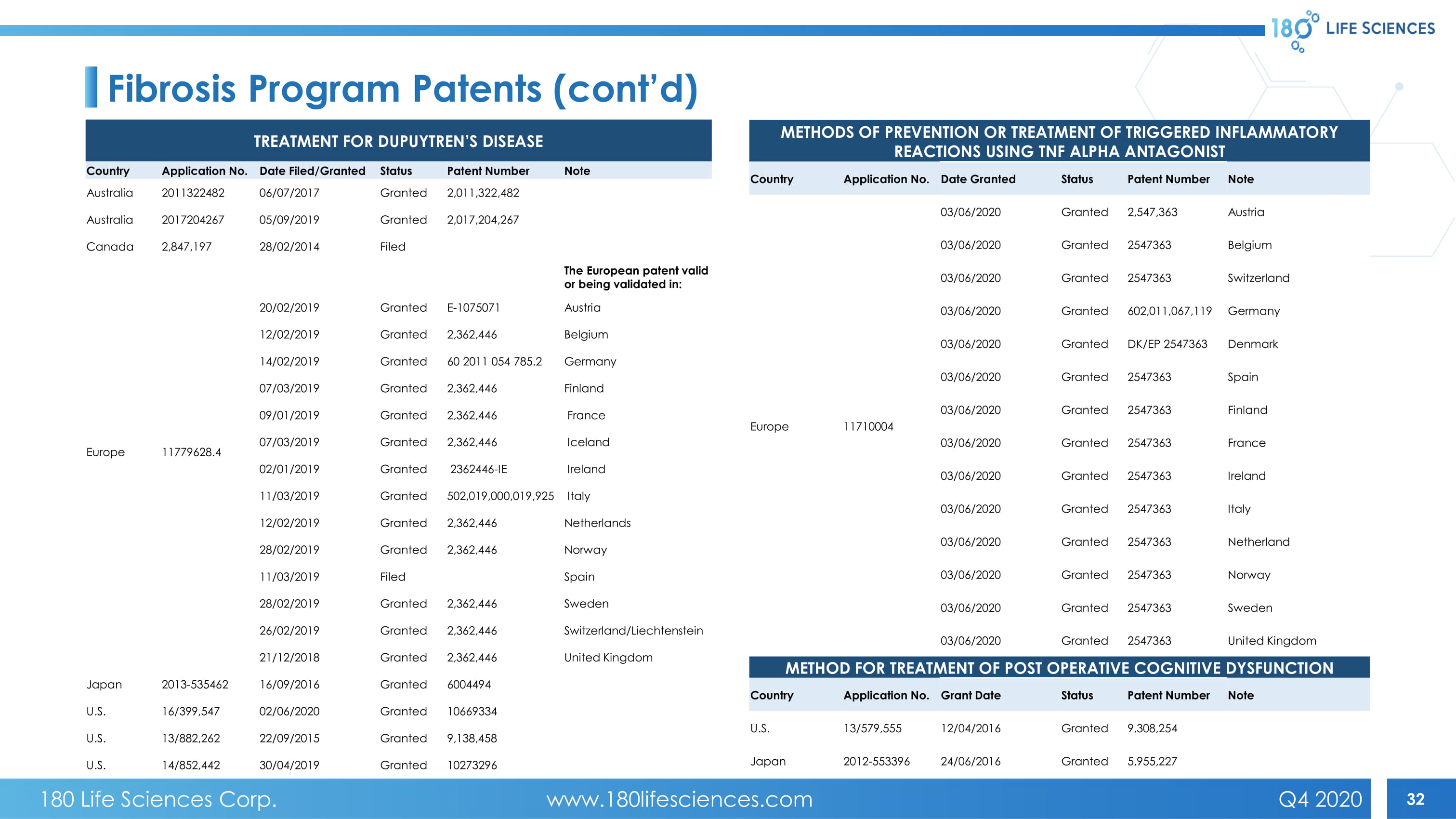
32 Fibrosis Program Patents (cont’d) TREATMENT FOR DUPUYTREN’S DISEASE Country Application No. Date Filed/Granted Status Patent Number Note Australia 2011322482 06/07/2017 Granted 2,011,322,482 Australia 2017204267 05/09/2019 Granted 2,017,204,267 Canada 2,847,197 28/02/2014 Filed Europe 11779628.4 The European patent valid or being validated in: 20/02/2019 Granted E - 1075071 Austria 12/02/2019 Granted 2,362,446 Belgium 14/02/2019 Granted 60 2011 054 785.2 Germany 07/03/2019 Granted 2,362,446 Finland 09/01/2019 Granted 2,362,446 France 07/03/2019 Granted 2,362,446 Iceland 02/01/2019 Granted 2362446 - IE Ireland 11/03/2019 Granted 502,019,000,019,925 Italy 12/02/2019 Granted 2,362,446 Netherlands 28/02/2019 Granted 2,362,446 Norway 11/03/2019 Filed Spain 28/02/2019 Granted 2,362,446 Sweden 26/02/2019 Granted 2,362,446 Switzerland/Liechtenstein 21/12/2018 Granted 2,362,446 United Kingdom Japan 2013 - 535462 16/09/2016 Granted 6004494 U.S. 16/399,547 02/06/2020 Granted 10669334 U.S. 13/882,262 22/09/2015 Granted 9,138,458 U.S. 14/852,442 30/04/2019 Granted 10273296 METHODS OF PREVENTION OR TREATMENT OF TRIGGERED INFLAMMATORY REACTIONS USING TNF ALPHA ANTAGONIST Country Application No. Date Granted Status Patent Number Note Europe 11710004 03/06/2020 Granted 2,547,363 Austria 03/06/2020 Granted 2547363 Belgium 03/06/2020 Granted 2547363 Switzerland 03/06/2020 Granted 602,011,067,119 Germany 03/06/2020 Granted DK/EP 2547363 Denmark 03/06/2020 Granted 2547363 Spain 03/06/2020 Granted 2547363 Finland 03/06/2020 Granted 2547363 France 03/06/2020 Granted 2547363 Ireland 03/06/2020 Granted 2547363 Italy 03/06/2020 Granted 2547363 Netherland 03/06/2020 Granted 2547363 Norway 03/06/2020 Granted 2547363 Sweden 03/06/2020 Granted 2547363 United Kingdom METHOD FOR TREATMENT OF POST OPERATIVE COGNITIVE DYSFUNCTION Country Application No. Grant Date Status Patent Number Note U.S. 13/579,555 12/04/2016 Granted 9,308,254 Japan 2012 - 553396 24/06/2016 Granted 5,955,227 180 Life Sciences Corp. www.180lifesciences.com Q4 2020
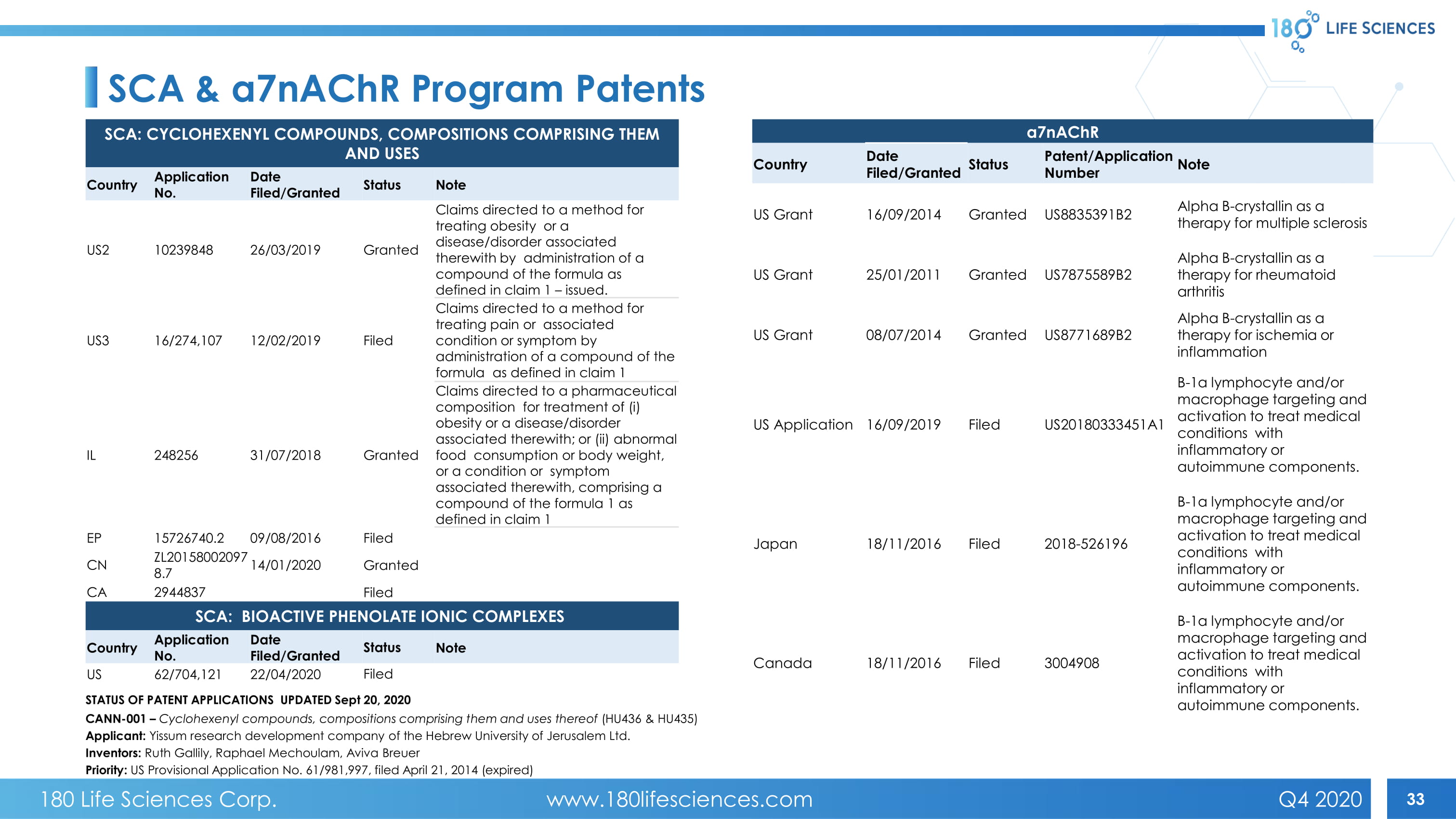
33 SCA & α7 nAChR Program Patents SCA: CYCLOHEXENYL COMPOUNDS, COMPOSITIONS COMPRISING THEM AND USES Country Application No. Date Filed/Granted Status Note US2 10239848 26/03/2019 Granted Claims directed to a method for treating obesity or a disease/disorder associated therewith by administration of a compound of the formula as defined in claim 1 – issued. US3 16/274,107 12/02/2019 Filed Claims directed to a method for treating pain or associated condition or symptom by administration of a compound of the formula as defined in claim 1 IL 248256 31/07/2018 Granted Claims directed to a pharmaceutical composition for treatment of (i) obesity or a disease/disorder associated therewith; or (ii) abnormal food consumption or body weight, or a condition or symptom associated therewith, comprising a compound of the formula 1 as defined in claim 1 EP 15726740.2 09/08/2016 Filed CN ZL20158002097 8.7 14/01/2020 Granted CA 2944837 Filed SCA: BIOACTIVE PHENOLATE IONIC COMPLEXES Country Application No. Date Filed/Granted Status Note US 62/704,121 22/04/2020 Filed α7 nAChR Country Date Filed/Granted Status Patent/Application Number Note US Grant 16/09/2014 Granted US8835391B2 Alpha B - crystallin as a therapy for multiple sclerosis US Grant 25/01/2011 Granted US7875589B2 Alpha B - crystallin as a therapy for rheumatoid arthritis US Grant 08/07/2014 Granted US8771689B2 Alpha B - crystallin as a therapy for ischemia or inflammation US Application 16/09/2019 Filed US20180333451A1 B - 1a lymphocyte and/or macrophage targeting and activation to treat medical conditions with inflammatory or autoimmune components. Japan 18/11/2016 Filed 2018 - 526196 B - 1a lymphocyte and/or macrophage targeting and activation to treat medical conditions with inflammatory or autoimmune components. Canada 18/11/2016 Filed 3004908 B - 1a lymphocyte and/or macrophage targeting and activation to treat medical conditions with inflammatory or autoimmune components. STATUS OF PATENT APPLICATIONS UPDATED Sept 20 , 2020 CANN - 001 – Cyclohexenyl compounds, compositions comprising them and uses thereof (HU436 & HU435) Applicant: Yissum research development company of the Hebrew University of Jerusalem Ltd. Inventors: Ruth Gallily , Raphael Mechoulam , Aviva Breuer Priority: US Provisional Application No. 61/981,997, filed April 21, 2014 (expired) 180 Life Sciences Corp. www.180lifesciences.com Q4 2020